Turner Syndrome
Turner syndrome is caused by monosomy X (karyotype 45,X) in which all or portions of one copy of the X chromosome are missing in females. Affected individuals can have a classic phenotype of short stature, broad chest, webbed neck, and low-set ears, but there is a wide range of phenotypes, and some individuals have less obvious physical findings.
Turner syndrome is associated with a wide range of medical conditions, the classic being gonadal dysfunction with sterility. Many individuals will also have abnormal liver enzyme levels. Biopsies in most cases show steatosis or steatohepatitis, but a subset can also have abnormal vasculature.6,7 The portal veins can be atrophic or absent. The liver can show nodular regenerative hyperplasia as well as focal hyperplasia, including multiple lesions. Patchy bile ductular proliferation that resembles biliary obstruction can also be seen. Some individuals will develop cirrhosis.6 In cases with cirrhosis, it is often unclear if the fatty liver disease or the vascular abnormalities, or both, were the driving injury leading to cirrhosis.
Hereditary Hemorrhagic Telangiectasia
Hereditary hemorrhagic telangiectasia (HHT) is also known as the Rendu-Osler-Weber syndrome and is an autosomal dominant genetic disorder leading to abnormal formation of blood vessels in various organs. The mutations lead to disruption of the transforming growth factor β (TGF-β) signaling pathway, and there are several subtypes based on the specific mutation. One of the subtypes is associated with SMAD4 mutations and juvenile type polyps of the intestinal tract. The subtype associated with ALK1 mutations is most likely to have liver disease.
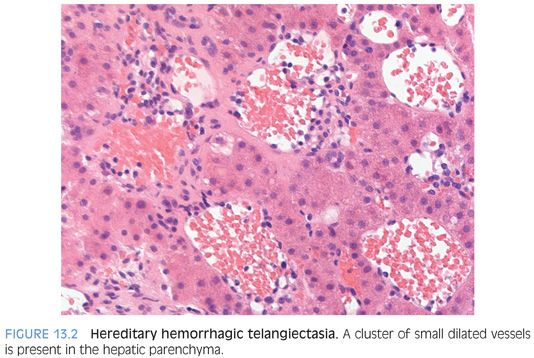
The telangiectasias can affect the face, oral mucosa including the tongue, and the mucosal lining of the nasal passages. Epistaxes (nosebleeds) are frequent and can be severe. The gastrointestinal tract is involved in about 20% of cases. Liver disease in the form of arteriovenous malformations (AVMs) can be seen in 50% to 75% of individuals8,9 and telangiectasias in 50%.8 Large AVMs can rarely present as high-output cardiac failure secondary to shunting of blood. Histologically, AVMs show clusters of larger caliber vessels with abnormal wall thickening. There can be localized areas of hemorrhage with fibrosis and hemosiderin laden macrophages. When there is localized hyperplastic response by the hepatocytes, a focal nodular hyperplasia can develop. Focal nodular hyperplasias are seen in about 5% of individuals.8 Imaging studies also commonly demonstrate dilatation of the large hilar hepatic arteries and intrahepatic shunts.
Liver lesions in most cases are multiple and include AVMs, focal nodular hyperplasias, telangiectasias (focal collections of dilated interanastomosing vessels in the portal tracts), and other vascular shunts. The liver is generally not biopsied if the diagnosis is known clinically because of the risk for bleeding. However, many cases that come to liver biopsy have not been diagnosed prior to the biopsy. In addition, some cases have only a single liver lesion8 and the diagnosis is not evident on imaging studies.
Histologically, HHT mass lesions show several different patterns. First, there can be focal nodular hyperplasias that are histologically similar to those in the sporadic setting.10 Second, there can be mass lesions with distinctive abnormal interanastomosing channels that are fed directly by portal veins (telangiectasias). The channels are thin-walled, and the endothelium has no atypia and a very low or absent proliferative rate (eFigs. 13.1 and 13.2). The channels dissect the hepatic parenchyma, leaving intact portal tracts and hepatic lobules (eFigs. 13.3 and 13.4). The hepatic lobules may show mild hyperplasia but do not show well-defined regenerative nodules. The lack of scarring, bile ductular proliferation, regenerative nodules, and thick-walled vessels separate these lesions from usual focal nodular hyperplasias. Mass-forming telangiectasias can be mistaken for hepatic adenomas on liver biopsy, depending on what gets sampled. In some cases, these single lesions present as a perfusion defect with only vague nodularity and an ill-defined mass. As a third type of nodular lesion, hepatocellular carcinoma has also been reported.11
Other changes besides mass lesions can include sinusoidal dilatation10,12 and nodular regeneration. Ischemic cholangiopathy has also been described.10,11 Microscopic telangiectasias can be present in the parenchyma outside the mass lesion. The telangiectasias are often less dramatic and show dilated vessels with less prominent anastomosis (Fig. 13.2).
HEPATIC INFLOW ABNORMALITIES
Portal Vein Thrombosis and Hepatoportal Sclerosis
Extrahepatic portal vein thrombosis can often be clinically occult and manifest with portal hypertension and ascites and a working clinical diagnosis of probable cirrhosis. There are many possible causes, but approximately half of all cases remain idiopathic. The patient should be worked up for thrombotic disorders. Other possible causes of portal vein thrombosis include abdominal inflammatory diseases that directly extend into the portal veins, causing inflammatory-related thrombosis. Examples include pancreatitis, appendicitis, and diverticulitis. Structural abnormalities from prior surgery can also predispose to thrombi. Finally, remote perinatal injury of the umbilical vein, for example, from sepsis or canalization, can also cause insidious thrombi that later present with noncirrhotic portal hypertension.
Idiopathic portal hypertension is the clinical term for cases where the liver is noncirrhotic and no portal vein thrombosis is seen. However, some cases of idiopathic portal hypertension can be related to prior, remote extrahepatic portal vein thrombosis that have become recannulated or are still there but radiographically occult.
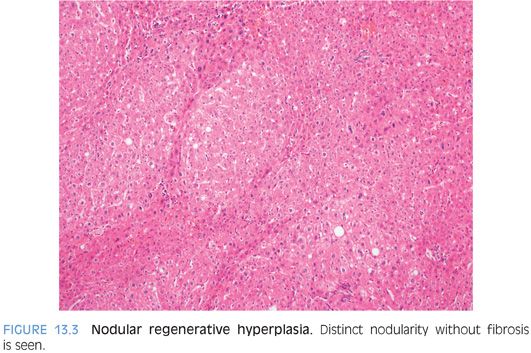
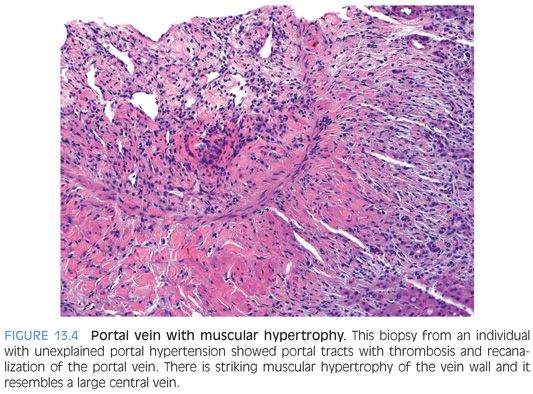
Peripheral liver biopsies can show mild and often subtle changes. The histologic constellation of findings is often referred to as hepatoportal sclerosis. In hepatoportal sclerosis, the portal veins can appear atrophic or be completely absent. In some cases, there can be increased numbers of small caliber portal veins, whereas in other cases, the portal vein branches can be “herniated” into the surrounding zone 1 hepatocytes. In other cases, there can be dense fibrous scars replacing the portal veins. The liver parenchymal often shows changes of nodular regenerative hyperplasia (Fig. 13.3). In rare cases, the intrahepatic portal veins can be associated with thrombosis. The thrombi can be remote and recannulated, and the vein wall can show striking muscularization (Fig. 13.4).
Portal vein thrombi can be associated with strictures and irregularities of the extrahepatic bile ducts, a finding called portal biliopathy. The changes can radiographically mimic cholangiocarcinoma. Portal biliopathy is more common in noncirrhotic than cirrhotic livers and is associated with extension of the thrombosis into the mesenteric veins. Portal biliopathy is thought to develop as collaterals around the thrombosed portal vein compress the extrahepatic bile ducts. The diagnosis is usually made radiographically, and biopsies are rare. However, when biopsied, the portal tracts can show ductular proliferation in keeping with extrahepatic bile duct obstruction.
Hepatic Artery Thrombosis
Hepatic artery blood flow is usually compromised by thrombi, but vasculitis is also a potential cause. Anastomotic strictures can also impair blood flow in cases of liver transplantation without causing full vascular occlusion.
The pathologic findings will vary depending on the location of the vascular injury and the clinical setting. The blood flow loss that results from thrombi of the extrahepatic arteries in a nontransplanted liver can often be compensated by retrograde blood flow from the portal circulation. However, injury to the smaller intrahepatic branches of the liver often leads to focal areas of infarction. In the transplant setting, early histologic findings in hepatic artery thrombosis include increased lobular spotty necrosis and increased numbers of mitotic figures with little or no inflammation. Long-term complications of hepatic artery compromise include chronic biliary tract disease, including strictures and duct loss.
SINUSOIDAL DISEASE
Sinusoidal disease can lead to impaired blood flow through the liver by physically blocking the sinusoids.
Sinusoidal Obstructive Syndrome
Several years ago, the term sinusoidal obstructive syndrome was chosen to replace the term veno-occlusive disease because the veins are not always histologically occluded. Although this fact was reasonably well understood by pathologists, who successfully using the term veno-occlusive disease for quite a long time, the decision was made to change the terminology and prevent future confusion. Sinusoidal obstructive syndrome is a fine enough term and should serve just as usefully as veno-occlusive disease.
Sinusoidal obstruction syndrome (SOS) is fundamentally caused by toxic or inflammatory injury to the endothelium of the sinusoids and/or central veins. Obliteration of the central veins is present in about 50% to 75% of liver biopsy cases of SOS and, despite the new name of SOS, can be a key histologic finding. Potential etiologies include herbal teas or remedies, bone marrow transplantation, and drugs used for chemotherapy. Total body or hepatic radiation therapy is also a potential cause. Many recent cases in the literature have been associated with oxaliplatin therapy for colon carcinoma. The frequency of chemotherapy-related SOS varies substantially in the literature. This reflects differences both in clinical protocols for chemotherapy as well as both underrecognition and overdiagnosis by pathologists. Mild and even focally moderate but patchy sinusoidal dilatation in resection specimens is very common, even in cases without histories of chemotherapy, and can sometimes be overinterpreted as SOS.
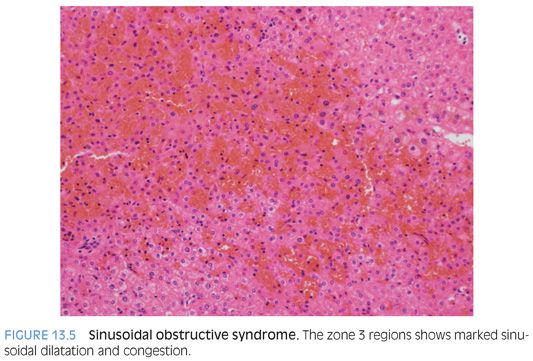
Histologically, the SOS pattern of liver injury from chemotherapy manifests by sinusoidal dilatation and congestion (Fig. 13.5). These changes can have a zone 3 distribution with “bridging congestion” on resections specimens or large biopsies (eFig. 13.5). Biopsies can show marked zone 3 sinusoidal congestion (eFig. 13.6). The zone 3 hepatocytes often show atrophy and occasionally small foci of acidophil bodies. Increased Kupffer cell iron accumulation can be present in longer standing cases. In addition, the central veins can show fibrous obliteration by loose and finely reticulated collagen, a finding that is often best seen on trichrome stain (eFig. 13.7). The lobules show little or no inflammation. Later, healed cases can show marked central vein scarring but have less prominent sinusoidal dilatation. Other findings can include changes of nodular regenerative hyperplasia and peliosis hepatis.
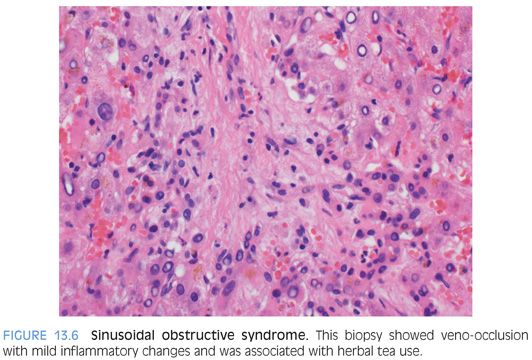
Histologic changes in SOS from non–chemotherapy-related drugs, for example, from herbal remedies, can have also mild lymphocytic inflammation in the scarred central veins (Fig. 13.6). The portal tracts also commonly show mild, predominately lymphocytic inflammation. Sinusoids can be dilated and congested, but this component is often less dramatic than that of chemotherapy-related SOS.
In wedge biopsies, the sinusoids can show artifactual dilatation, often dramatic, due to the effect of cautery (eFig. 13.8). The sinusoidal dilatation can sometimes even retain a zone 3 pattern, although often the changes show no zonal associations. The sinusoids typically do not contain blood but instead are empty or have a grey amphophilic material (eFig. 13.8).
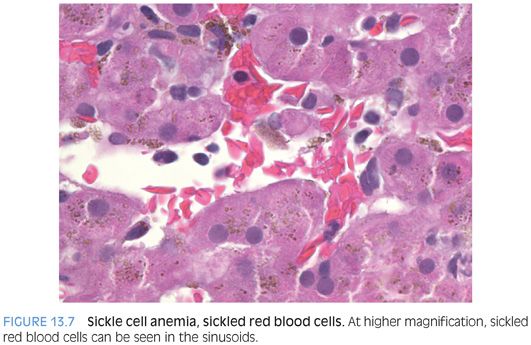
Sickle Cell Disease
Sickle cell disease can manifest in the liver with different patterns of injury. The most common pattern is iron overload secondary to transfusions, where biopsies are performed in most cases to obtain tissue for quantitative iron analysis. Biopsies in these cases also provide important fibrosis staging information. Iron stains typically show moderate to marked hepatocellular and Kupffer cell iron accumulation. The sinusoids often show congestion (eFig. 13.9), and erythrophagocytosis is found in most cases with sufficient searching (Fig. 13.7). Some cases may also show mild biliary tract obstructive type changes because passage of small biliary stones is common. With improved medical management and improvements in lifespans, liver cirrhosis secondary to the iron overload is of increasing clinical concern (eFig. 13.10). The fibrosis pattern progresses from portal fibrosis to bridging fibrosis to cirrhosis. Approximately 10% to 20% of individuals will develop liver cirrhosis.13,14