Fig. 9.1
Large bladder stones arising in a patient with neurogenic bladder and recurrent UTI
The mean time to diagnosis of bladder stones following enterocystoplasty is approximately 5.5 years, and the most common presenting complaint is UTI [23, 25]. A urease-splitting organism was found in 89 % positive urine cultures and not unexpectedly, the proportion of struvite stones was the same [23]. A complete discussion of urolithiasis in congenital neuropathic patients, including management, can be found in Chap. 15 (Monga) in this textbook.
Following complete evacuation of the stone fragments, regular bladder irrigation can be employed to prevent stone recurrence. However, it is important to be aware that irrigation has been found to fail in 33–44 % of patients, who experience recurrence of stones [23]. Stone composition is most commonly carbonate apatite complexed with struvite, followed by ammonium acid urate [27]. All patients from the DeFoor stone series had chronically alkaline urine with a median urine pH of 7.5 and common bacterial pathogens identified on urine culture were Proteus and Klebsiella species. It is for this reason that suppression of UTI if possible may also be a very important part of decreasing stone formation in addition to bladder irrigation. Suppression of UTI may fall into a variety of categories, including finding strategies to rid a patient of chronic indwelling catheters, or treatment of abnormal bladder compliance.
Patients with neurogenic bladder are also at risk for upper tract stone formation. For instance, upper tract stones effect 30 % of tetraplegic patients regardless of history of prior urological surgery and is attributed to both immobilization and VUR of infected urine [28]. In the reconstructed bladder, typical retrograde treatment of upper tract stones may not be possible due to bladder neck procedures or a history of ureteral re-implantation and percutaneous nephrolithotomy must be performed in these cases (Fig. 9.2).
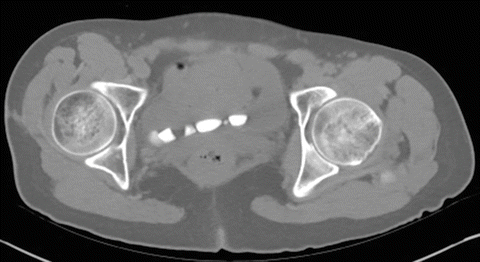

Fig. 9.2
Stones in the distal ureter that has been re-implanted across the trigone. This anatomy can make conventional retrograde approaches challenging or impossible
The workup for nephrolithiasis and/or bladder stones is variable. Renal ultrasound accompanied by a plain film of the kidneys, ureter, and bladder (KUB) will likely find most cases of large bladder and renal calculi. The most definitive test for urinary stones, however, is computed tomography (CT) scan, since uric acid stones may be radiolucent and missed in a KUB. A CT scan of the abdomen and pelvis can be done without contrast for patients with a dye allergy or renal insufficiency or it can be done with intravenous contrast (in order to obtain excretory phase images), which gives more information about the drainage of the kidneys and ureters, as well as identifying scars and evidence of ongoing pyelonephritis. A CT scan is indicated when new or worsening UTI is accompanied by hematuria.
Vesicoureteral Reflux
VUR may lead to recurrent pyelonephritis episodes in patients with otherwise asymptomatic bacteriuria.
There is contradictory evidence about the need to treat VUR at the time of augmentation cystoplasty.
The importance of VUR in adults is debatable. However, in patients with neurogenic bladder and reconstructed bladders it may be important to treat. New or worsening VUR can indicate worsening bladder compliance and a need for re-evaluation of pressures with a cystometrogram (urodynamics) (Fig. 9.3). VUR may complicate UTI because otherwise asymptomatic bacteriuria may ascend and cause febrile UTI and systemic symptoms. A large and dilated refluxing system may also act as a reservoir of static urine, where infection can arise. Some studies show that augmentation cystoplasty alone resolves or downgrades nearly all VUR in both adult and pediatric populations by restoring a low-pressure system [29–31]. These findings are controversial, however, and some recent studies have found opposite results. In one study of children undergoing augmentation cystoplasty with small bowel, low-grade VUR persisted in only 10 % of patients, however, high-grade VUR persisted in 47 %, and about half of these patients went on to experience pyelonephritis if they did not have treatment of VUR at the time of augmentation cystoplasty [32]. Conversely, patients who undergo ureteral re-implantation at the time of augmentation cytsoplasty were found to have complete resolution of high-grade VUR and a markedly decreased incidence of UTI [33]. Based upon these findings, authors recommended, ureteral re-implantation for high-grade VUR at the time of augmentation cystoplasty. Interpreting these contradictory results is difficult, however, and consideration should be given to the fact that treating VUR with ureteral re-implantation after augmentation is a much harder task than at the time of augmentation cystoplasty.
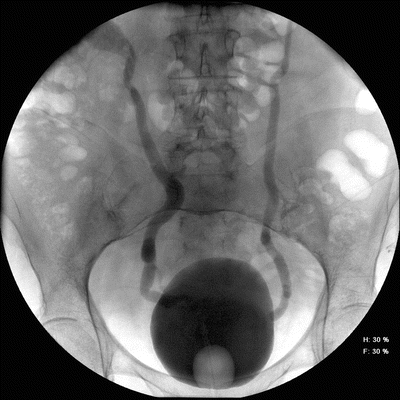

Fig. 9.3
Severe bilateral vesicoureteral reflux in a patient with poor compliance from neurogenic bladder
In follow-up regimens of patients with neurogenic bladder, it is often argued that patients should have annual or regular cystograms to monitor for VUR. This recommendation may be unnecessary since there is very little data to support treatment of VUR in asymptomatic adults, other than for evidence of ascending infections that cause pyelonephritis episodes. A targeted approach in individuals who are having febrile UTI or evidence of pyelonephritis will save patients from many unnecessary cystograms and associated risks, such as induction of urosepsis or catheter trauma.
Poor Bladder Compliance and UTI Risk
High-pressure bladder dynamics and poor compliance can be a major component of difficult to treat UTI
Measures to improve bladder dynamics lessen UTI rates
Lapides popularized the concept that high intravesical pressure and bladder over distention is responsible for increased UTI risk. The postulated mechanism is that when the bladder is subjected to periods of reduced blood flow, its susceptibility to bacterial invasion is increased [34]. There is a substantial amount of indirect evidence supporting this theory. For instance, urodynamic testing in infants with UTI but without VUR showed high voiding detrusor pressures of 40–100 cm, suggesting that the high-pressure voiding itself, rather than an anatomic defect, predisposed these infants to UTI [35].
The link between high-pressure voiding and UTI is further illustrated by a decrease in the incidence of UTI through interventions that increase bladder capacity and decrease detrusor tone. For example, surgical release of spinal cord tethering has been found to increase bladder capacity and decrease detrusor leak point pressures, with a corresponding drop in febrile UTI [36]. Similarly, improved bladder capacity and decreased bladder pressures observed in patients with sacral nerve modulator implants, placed immediately after SCI, and has also been associated with a decreased incidence of UTI [37]. Other evidence supporting this concept is in patients with neurogenic detrusor overactivity due to multiple sclerosis (MS) or SCI who received detrusor injections of 300 U of onabotulinum toxinA. In this study, the number of UTIs over 6 months decreased from a mean of 1.75 to 0.2. Interestingly, those patients with persistent symptomatic urinary infections also demonstrated less improvement in their urodynamic parameters, reflecting the idea that improved reservoir capacity and lower pressures are protective against UTI [38]. In contrast to this study, however, it is important to note that UTI risk was higher in patients treated onabotulinum toxinA, in the DIGNITY trial. This increased risk was likely due to patients with MS starting intermittent catheterization. As part of the same study, the patients with SCI who were already performing catheterization did not have a decrease in UTI, but UTI was only defined as positive cultures, which were routinely obtained during follow-up and there was no quantification of symptomatic UTI.
When compliance is felt to be a major issue, then a urodynamic study (cystometrogram) is warranted. Even in patients with previous bladder augmentation a low capacity and poor compliance bladder may be an issue. In fact, up to 9 % of patients with a history of pediatric augmentation cystoplasty require re-augmentation [25, 26, 39]. A bladder journal documenting the patient’s functional volumes can also provide vital information about their capacity and identify patients who might benefit from urodynamics.
Anatomic Problems
Anatomic problems that need surgical correction are commonplace in patients with reconstructed bladders.
Increased UTI rates can be caused by problems such as ureteropelvic junction obstruction or stenosis of the distal ureter at the site of previous ureteral re-implantation.
Among patients who have undergone bladder reconstruction, in particular augmentation cystoplasty, 34–40 % can be expected to require an additional urological procedure in the future. The most common surgery is cystolithalopaxy, accounting for 25 % of interventions post-augmentation, with a median of two occurrences of stones [25, 26]. In regard to major revision surgery, urinary diversion is required in 5 %, repair of bladder rupture in 3 %, treatment of small bowel obstruction in 3 %, and re-augmentation in 9 % due to issues with persistent incontinence, isolated upper tract changes, and detrusor pressure of >30 cmH2O [25, 26, 39]. Revision rates for a catheterizable channel have also been reported to be as high as 20 % [40], and in our experience up to 50 % of patients require revision surgery for tunneled catheterizable channels [21]. While many of these operative problems do not lead to increasing UTI, they serve to emphasize that anatomic problems which need to be addressed with surgery are commonplace after bladder reconstruction.
When UTI begin or worsen in patients with previous bladder reconstruction or augmentation, it is important to fully evaluate any anatomic reasons that could be contributing. One of the most important considerations is kidney obstruction from previous ureteral surgery. While ureteral re-implant surgery to treat VUR has a very low stenosis rate long term, this may not be true in neurogenic bladder patients. The neurogenic bladder is fundamentally quite different; it is thickened and fibrotic, lacking normal elasticity and may have been adversely affected by years of chronic cystitis. All of these factors can lead to recurrent VUR or stenosis. As mentioned above, VUR can be a cause of febrile UTI in patients due to pyelonephritis and is evaluated with a cystogram. Ureteral stenosis is evaluated with a nuclear medicine lasix renogram. It is often difficult to interpret the results of lasix renograms, however, given that many patients who have had chronic hydronephrosis may exhibit some degree of delayed emptying. In cases that are equivocal, we often perform retrograde or antegrade ureteral pyelography.
Another anatomic problem that we have seen arise in patients with neurogenic bladder and previous bladder reconstruction is ureteropelvic junction obstruction (UPJO). There is a small incidence of UPJO associated with VUR that has been established in pediatric urology. These cases of UPJO may be due to this association, or they may arise secondary to fibrosis surrounding the ureter from ascending infection, stones, or previous manipulation (Fig. 9.4). Other causes are redundancy arising from chronic hydronephrosis secondary to a history of VUR and high-pressure bladder dynamics. Ureteral redundancy can cause a kinking effect in the ureter and in some cases can lead to a functional obstruction. An evaluation can be performed with a nuclear medicine lasix renogram, potentially in combination with retrograde ureteropyelograms [41].
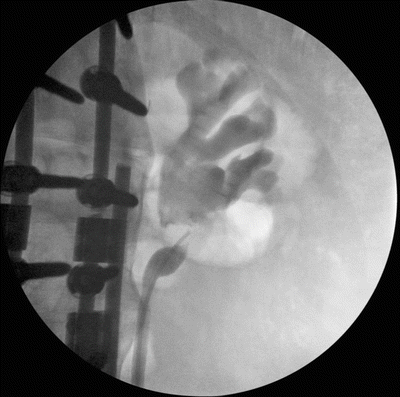

Fig. 9.4
Ureteropelvic junction obstruction in a patient with spinal cord injury that may have arisen from congenital issues, scarring, or ureteral redundancy
Finally, the shape of the reconstructed bladder itself can be a predisposing factor for recurrent infections, as when the bladder enlarges and gains a saccular shape. This can create a situation in which portions of the bladder are not drained to completion with intermittent catheterization, resulting in urinary stasis. Such a situation can be diagnosed using cystogram before and after catheterization, and may be a diagnosis of exclusion after all other sources of urinary infections has been ruled out. One approach to managing an incompletely draining reconstructed bladder is scheduled manual irrigations through a catheter followed by active aspiration of the irrigant.
Clean Intermittent Catheterization
Noncompliance with catheterization schedules can increase UTI rates.
There is contradictory evidence to suggest hydrophilic catheters offer any advantage.
A regular schedule of CIC has been shown to be safe and effective in the long term and can avoid bladder distention and high intraluminal pressures, allowing bacteria introduced by CIC to be neutralized. The rate of UTI and bacteriuria, as well as other urologic complications are lower in patients who use CIC compared to indwelling catheters, and thus is the recommended option [42].
In a bladder with normal capacity and compliance, the frequency of catheterization is every 4–6 h, with an aim to maintain bladder volumes <500 mL. However, given the varied bladder compliance and capacity in patients with neurogenic or augmented bladders, the regimen must be individualized on a case-by-case basis. Typically 12–16F catheters are used for both adult men and women, with larger catheters employed in augmented bladders requiring irrigation. Clean, as opposed to sterile, intermittent catheterization does not pose an increased risk of symptomatic UTI in SCI patients and has significant cost and time saving benefits [43]. When it comes to catheter selection, there is contradictory evidence about the use of hydrophilic catheters, with some studies showing lower rates of infection and hematuria compared to non-hydrophilic catheters [44].
Of adult patients who have been instructed on CIC, only about 2/3 are compliant with at least 80 % of the initial recommendation at 1 year [45]. Noncompliance with catheterization leads to overflow incontinence, which in itself can contribute to UTI risk [46, 47] as well as higher rates of UTI due to more time spent with elevated intravesical pressure. A very simple way of assessing catheterization volumes, leakage, and patient compliance is to have patients keep a bladder journal for several days.
Prevention Strategies for UTI
Once modifiable problems with the urinary tract have been eliminated or treated and UTI remains as a persistent issue, then treatment should focus on prevention of infections. Prevention strategies are best employed in a very systematic and stepwise fashion starting with therapies that minimize chronic antibiotic use. Some of these strategies are in development such as bacterial interference, while others are commonly used. The evidence underlying these strategies is often contradictory or lacking; however, they are often tried in an effort to control UTI in patients who are commonly very affected by their UTI.
Bladder Irrigations
Bladder irrigations with NS or water are safe and effective in evacuating bacteria, associated biofilms, and mucous.
Irrigation with antibiotic solutions can also be used; however, their superiority to NS or water irrigations has not been established.
The use of gentamicin bladder irrigation in augmented bladders is common [48] and has been demonstrated to be safe with little systemic absorption [49]. When compared to a control group of no treatment, both neomycin/polymyxin B and kanamycin/colistin irrigations have demonstrated efficacy in reducing incidence of bacteriuria in SCI patients with neurogenic bladder [50, 51]. However, when neomycin-polymyxin irrigations were compared to irrigations with sterile saline or acetic acid, there were no differences in bacteriuria or pyuria [52]. Additionally, bladder irrigation with sterile NaCl 0.9 % versus tap water has shown no difference in the incidence of positive urine cultures [53]. This observation lends support to the theory that the mechanical effect of a washout rather than the antiseptic properties of the agent being instilled is what ultimately helps in controlling the bacterial burden in patients.
Acidic bladder washout solutions have been shown to be superior to saline in reducing struvite crystals, however whether this decreases stone formation is unclear [54]. Approaches to stone prevention proposed by DeFoor et al. include an early regimen of regular low-volume bladder irrigation, with a transition to high-volume bladder irrigation regimen after stone development and treatment. In the event of recurrent stone formation on high-volume irrigation, 20 % urea solution irrigation is proposed, although it is yet to be proven effective [27]. Compliance is the main barrier to the efficacy of irrigations in UTI prevention. A 4-year study of children with ileocystoplasty demonstrated with close monitoring of compliance with an irrigation regimen, UTI can be virtually eliminated and stone incidence can be decreased to 7 % [55].
Bowel Management
The link between functional constipation and recurrent UTIs has been well documented, especially in the pediatric population. A recent study of children with myelomeningocele found a correlation between the intestinal production of methane, prolonged orocecal transit time, and a higher incidence of UTIs [56]. In another study of pediatric patients with lower urinary tract dysfunction, almost half of patients with constipation also experienced UTIs [57]. One proposed mechanism is that the distended rectum in a constipated child produces obstruction of the bladder outflow. Another hypothesis is that intestinal stasis causes overgrowth of bacteria, which then translocates to the genitourinary tract. Approaches to improved bowel management include an oral regiment of laxatives, colonic stimulants, and stool softeners, retrograde enemas, or antegrade enemas via a continent appendiceal stoma. In the adult population of patients with neurogenic bowel, digital stimulation, manual disimpaction, and suppositories are additional approaches to attain regularity and minimize direct contamination of the urethra that can be the result of encopresis.
Prophylactic Antibiotic Regimens
Prophylactic antibiotics may decrease UTI rates with the significant downside of higher rates of resistant infection.
In SCI patients, the use of long-term systemic antibiotic prophylaxis achieves only modest protection against UTI, at the expense of an increase in antimicrobial resistance and adverse drug reactions [58, 59].
However, prophylactic antibiotic treatment needs to be considered in patients with recurrent UTIs, high-pressure bladders, and a dilated upper urinary tract which predispose patients to urosepsis. Some authors recommend a 3-month course of prophylactic antibiotics if UTIs persists after treatment of an established infection [60].
Commonly utilized prophylactic antibiotic agents include oral trimethoprim and sulfamethoxazole, nitrofurantoin, and ciprofloxacin. Often these are used at one half to a quarter of a daily dose for 6 months. In pediatric patients on CIC for neurogenic bladder, a 10 % decrease in bacteriuria and a 50 % decrease in symptomatic UTIs were observed on nitrofurantoin prophylaxis compared to placebo. Bacterial species responsible for bacteriuria were altered in the nitrofurantoin group, with E. coli being replaced by resistant Klebsiella and Pseudomonas species with a tripled rate of resistance [61]. In contrast, antimicrobial prophylaxis in adult patients on CIC did not affect the incidence of symptomatic UTI compared to placebo [62]. A meta-analysis of adult patients with SCI revealed that antimicrobial prophylaxis did not significantly decrease symptomatic infections. Prophylaxis was associated with a reduction in asymptomatic bacteriuria among acute patients (<90 days after SCI), with the finding that one patient would require 3.7 weeks of treatment on average to prevent one asymptomatic infection. Prophylaxis resulted in an approximately twofold increase in the proportion of antimicrobial resistant bacteria [58].
Cyclic High-Dose Antibiotics for UTI Prophylaxis
Another antibiotic-based suppressive regimen is keeping patients on weekly cyclic high-dose antibiotics.
An observational prospective study evaluating the efficacy of alternating once-weekly oral cyclic antibiotic (WOCA) regimen over 2 years to prevent UTI in SCI adult patients with neurogenic bladder undergoing CIC, revealed a significant decrease in the incidence of UTI. Before intervention, there were 9.4 symptomatic UTIs per patient-year, including 197 episodes of febrile UTI responsible for 45 hospitalizations. Under the WOCA regimen there were 1.8 symptomatic UTI per patient-year, including 19 episodes of febrile UTI. No severe adverse events and no new cases of colonization with MDR bacteria were reported [59]. In our anecdotal experience, the WOCA regimen appears to be quite helpful for some patients that struggle with recurrent symptomatic UTI and we often offer this over daily low-dose prophylaxis. Complications and limitations to this strategy are the development of Clostridium difficile colitis and baseline-resistant UTI.
Cranberry
Although cranberry extract is commonly used to suppress UTI, the evidence is contradictory and a recent Cochrane review found little evidence to support its use.
The postulated mechanism of action of cranberry is the effect of a proanthocyanidin molecule contained within cranberries that impairs bacterial adherence to the urothelium, thereby reducing the biofilm load of both Gram-negative and Gram-positive bacteria [63]. In a randomized placebo-controlled study with crossover design of SCI patients with neurogenic bladder, cranberry extract administration reduced the frequency of UTI to 0.3 per year, compared to 1 UTI per year on placebo. Subjects with a glomerular filtration rate (GFR) greater than 75 mL/min received the most benefit [64]. In contrast, a 1-year study comparing cranberry extract (36 mg proanthocyanidins BID) versus placebo in adult MS patients with urinary disorders demonstrated no difference in incidence of UTI [65]. Similarly, a cross-over study of patients with neurogenic bladders due to SCI and requiring catheterization randomized to 400-mg cranberry tablets TID for 4 weeks or placebo demonstrated no difference in urinary pH, bacteriuria, or pyuria [66]. In the 2013 Cochrane review, cranberry products were not found to significantly reduce the risk of repeat symptomatic UTI compared to placebo for any subgroups, including women with recurrent UTIs and patients with neurogenic bladder or SCI [67].
d-Mannose
d-Mannose in randomized studies of limited populations is as effective as daily antibiotics in suppressing UTI rates.
d-Mannose is a naturally occurring sugar used by the human metabolism in protein glycosylation. It has been shown to bind to and block FimH adhesin on the fimbria of enteric bacteria as well as bind to urothelial glycoprotein receptors, thereby inhibiting bacterial adherence. When used for a 6-month interval in women with recurrent UTI, the incidence of UTI in the d-mannose and nitrofurantoin groups was 15 % and 20 %, respectively, compared to 60 % in the group receiving no prophylaxis [68]. d-mannose has yet to be evaluated in neurogenic and augmented bladders.
< div class='tao-gold-member'>
Only gold members can continue reading. Log In or Register to continue
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree




