Fig. 10.1
Stepwise approach to troubleshoot obstruction in catheterizable channels
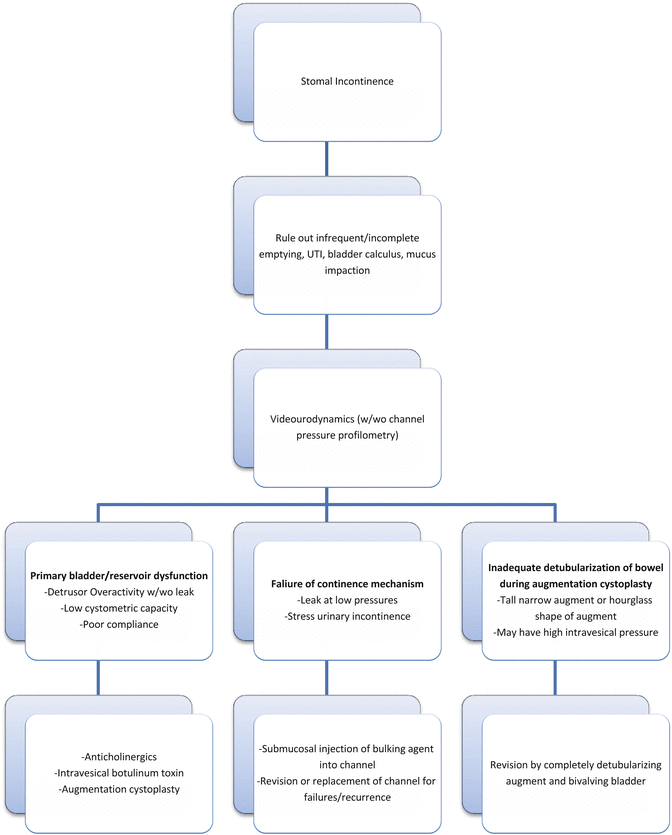
Fig. 10.2
Stepwise approach to troubleshoot incontinence through catheterizable channel
Obstruction
Inability to catheterize the channel requires emergent intervention in patients whose bladder neck has been surgically closed or narrowed. In patients with a patent urethra and bladder neck, the situation is less dire. The patient, caregiver or healthcare provider can initiate intermittent urethral catheterization or place an indwelling urethral catheter until the channel obstruction can be addressed. Still, channel access should be re-established within a couple of days; it has been our experience that earlier intervention is more successful than delayed intervention.
The first step in obtaining channel access is a gentle attempt at catheterization using a well-lubricated catheter of the same or lesser size as routinely used by the patient. If the urethra is patent, a urethral catheter should be placed before attempting to catheterize the channel because a distended bladder can complicate catheterization of the channel in a couple of ways: (1) the channel can become kinked as the bladder distends and moves closer to the stoma; (2) the distended bladder will tighten the Mitrofanoff tunnel. If an initial attempt at channel catheterization is unsuccessful, then a second attempt with a hydrophilic guidewire can be helpful. However, before causing too much trauma, the urologist should have a low threshold to perform endoscopy of the channel and place a catheter over a wire. The small diameter of the catheterizable channels usually means that endoscopy should be done with a flexible pediatric cystoscope, flexible hysteroscope, or a flexible adult ureteroscope. A rigid pediatric cystoscope can be better suited for cases of stomal stenosis. When the urethra is patent, endoscopic attempts can be combined with transurethral cystoscopic guidance.
Dilation of the stoma or channel can be performed over the wire. We prefer to do this with a series of successively larger straight catheters over the wire. Dilation should not exceed the normal size of the channel—excessive dilation risks injury to the rest of the channel and the continence mechanism. Welk et al. have reported the use of steroid lubricant after stomal dilation to keep the stoma patent, although the durability of this treatment is not known [8]. Endoscopic incision of channel stricture has been described although we prefer dilation to incision because of the fragile nature of catheterizable channels and the risk of injury to adjacent bowel. Once a catheter is placed, it should be left in place for 1 or 2 weeks following which the catheter can be removed and patient asked to resume CIC.
If initial endoscopic attempts fail and bladder drainage cannot be obtained through urethral catheterization, then suprapubic tube (SPT) placement should be considered. Although percutaneous SPT placement can be performed faster and with local anesthesia, the reconstructed lower urinary tract in many of these patients may necessitate open cystotomy and SPT placement. If the surgeon is facile with ultrasound-guided SPT placement, then this is a less invasive alternative to safely placing an SPT when adjacent bowel is of concern. Definitive management can then be planned on an elective basis, keeping in mind that the solution can be as simple as stomal revision or require removal and replacement of the channel. Mickelson et al., have described an “L-stent” which can be used for refractory stomal stenosis. This can act as a temporizing measure until surgical revision, or in some cases, obviate the need for surgery altogether [17].
Definitive management is indicated if endoscopic attempts fail or if obstruction recurs despite minimally invasive interventions. Stomal stenosis can be corrected by excising the scarred tip and advancing a local skin flap into a spatulated, healthy distal segment, as a Y-V plasty. We perform this with a wire in the channel when possible and dissect circumferentially down to fascia in order to mobilize a healthy segment of channel. This is a relatively minor procedure that can be accomplished in the ambulatory setting. Buccal mucosa graft can also be used for stomal revision as described by Radojicic et al. [18]. If the channel stricture is below the level of fascia, laparotomy is required for revision; unfortunately, this can be hard to predict preoperatively so we prepare all patients for the possibility of a laparotomy.
Upon laparotomy, identification of the stenosed portion of the channel is usually straightforward—even the outer wall of the channel is ischemic and fibrotic. Depending on the length of the involved segment, the channel can be revised by interposition of a segment of bowel or the entire channel may need to be removed and replaced (see Fig. 10.1). If only the distal portion of the channel is fibrosed and the continence mechanism is unaffected, then a new channel can be fashioned (e.g., Monti) and anastomosed end-to-end to the healthy end of the existing channel. If the entire channel is fibrotic, it should be resected and a new channel created. Ideally, the new channel is tunneled in the native bladder. However, if bladder size or chronic cystitis and mucosal inflammation preclude formation of a good detrusor tunnel, then a channel using an alternative continence mechanism must be entertained. A rather simple salvage procedure in this case is to add an ICCC to the native bladder or augment, using the ileocecal nipple valve for continence. A more challenging alternative that we reserve for cases in which the ileocecal valve is not available is the intussuscepted ileal flap valve [19].
Incontinence
In a patient with stomal incontinence, it is important to rule out causes such as infrequent catheterization, incomplete bladder emptying, UTI, bladder calculus, or mucus impaction. In the absence of such causes, urodynamic studies (UDS) can help determine the etiology of incontinence. Pressure profilometry of the channel can be performed at the time of UDS; however, there is no standard cut-off below which the value might be considered abnormal. If urethral access is present, the UDS catheter can be placed transurethrally and patient filled and stressed to demonstrate leakage with stress or with bladder filling to a specific volume. Low-pressure leakage, stress urinary incontinence, and the absence of detrusor overactivity (DO) would implicate the continence mechanism as the cause of incontinence. On the other hand, the presence of DO, leak with DO, or impaired compliance implies primary bladder pathology. Treatment can then be directed toward rectifying the cause of the incontinence (Fig. 10.2). If detrusor overactivity is identified as the cause of incontinence, conservative measures such as anticholinergic medications and intravesical Botulinum toxin-A should be tried first. If the patient does not respond to such measures, he/she may be a candidate for augmentation cystoplasty or revision of an existing augmentation cystoplasty.
Endoscopic injection of bulking agents into the channel can be an effective, minimally invasive method to address failure of continence mechanism. Biomaterials that have been used for this purpose include collagen, PDMS, and dextranomer/hyaluronic acid injection (Deflux®). Prieto et al. have reported success rate of 71 % after a single injection and 79 % after two injections of Deflux in patients who were candidates for surgical revision for stomal incontinence. The mean follow-up duration in this series was 1 year [20]. Longer term studies are necessary to assess the durability of response with bulking agents. If incontinence does not respond to bulking agents, surgical intervention may be necessary. Revision of the existing continence mechanism, or removal and replacement of the entire channel with tunneled detubularized ileum (Monti), ICCC, or intussuscepted ileal flap valve as discussed in the earlier section can be considered. Yachia and Erlich have described the technique of creating a continent stoma by cross-wrapping non-detached strands of rectus muscle around the efferent channel. In their series of 17 patients, 100 % continence was reported after a mean follow-up duration of 32 months. Although described for primary creation of a continent reservoir, this technique can also be used as a salvage maneuver in cases of failure of continence mechanism [21].
< div class='tao-gold-member'>
Only gold members can continue reading. Log In or Register to continue
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree




