Fig. 1
Basic techniques of elastography. Differences between strain and shear-wave elastography
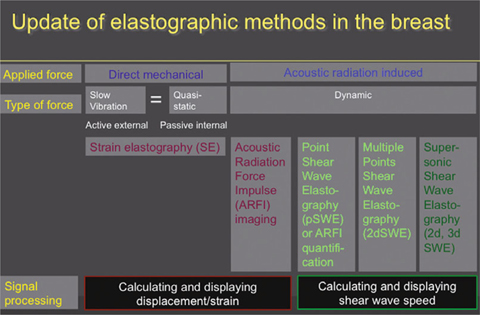
Fig. 2
Indications for Breast Ultrasound
A list of updated recommendations pertaining to indications is given in Table 1. US is the first-line imaging technique for women <40 years presenting with symptoms or clinical signs. In the presence of a suspicious lesion, US is the method of choice to guide core biopsy to harvest tissue. US-guided vacuum-assisted biopsy (VAB) is used increasingly to diagnose intraductal lesions, small architectural distortions, and borderline lesions; to complete preoperative staging in patients with extensive ductal component; and for therapeutic excision. Stereotactic-guided VAB is the method of choice by which to sample screen-detected microcalcifications and architectural distortions not seen on US. In the dense breast, the combination of US and screening mammography improves cancer detection considerably compared with mammography alone but with an increase in biopsy rate. The additional diagnostic yield of US after negative mammography is 3.2:1,000 women with dense breasts. Intraoperative surgeon- performed US focuses on accurately defining the resection segment or sector and margin analysis of the resection specimen. MRI is useful preoperatively to assess the extent of ipsilateral disease and exclude contralateral breast cancer, particularly for women at increased risk of mammographically occult disease. Second-look US can detect up to 50% of MR-enhancing cancers with negative mammography [5–8].
Table 1
Updated indications for high-resolution ultrasound (HRUS)
– | Differentiation of cysts and solid tumors |
– | Differentiation between solid, benign, and malignant lesions |
– | Characterization of palpable abnormalities |
– | Assessment of mammographic screening abnormalities |
– | Dense breasts showing reduced mammographic sensitivity |
– | Diagnosis and follow-up of women with benign breast disease or risk lesions |
– | During pregnancy or lactation |
– | Significant nipple discharge |
– | Under hormonal replacement therapy |
– | Inflamed breast and abscess formation |
– | Extended screening for high-risk patients |
– | Second look after magnetic resonance mammography |
– | Guidance of interventional procedures, such as fine-needle aspiration, core biopsy, diagnostic and therapeutic vacuum biopsy, preoperative tumor localization, axillary lymph node biopsy |
– | Preoperative lesion staging; skin and nipple distance for planning breast-conserving surgery, mastectomy, or oncoplastic reconstruction with implants; assessment of multifocality, multicentricity, intraductal extension, lymph node changes, and contralateral lesions |
– | Preoperative staging and follow-up under neoadjuvant chemotherapy |
– | Surveillance after breast-conserving therapy |
– | Silicone implants |
Examination Technique
The International Breast Ultrasound School (IBUS) and American College of Radiology (ACR) guidelines for breast US examination advise a systematic, comprehensive, and reproducible examination technique, followed by documentation, description, reporting, classification, and recommendation. The examination starts with proper positioning of the patient in a supine or anterior oblique position depending on breast volume, with elevation of the ipsilateral arm. Positioning should result in a maximum flattening of the breast portion being examined. Automated tissue optimization and focal-zone and field of view (FOV) settings should be optimized before scanning, with the transducer perpendicular to skin. A minimum of two scan planes is recommended in wholebreast US. Image analysis of a detected lesion or pseudolesion requires rotation of the transducer over the entire lesion using changing compression intensities and angulations. Radial imaging of adjacent ducts is mandatory to assess ductal extensions. BI-RADS descriptors and further criteria of additional elastography, 3D tissue criteria, vascularization, and associated lymph node morphology characterize a state-of-the-art lesion assessment by US. The o’clock position and distances to skin and nipple describe the exact localization of a lesion within the volume of the breast. Indication of palpability and imaging correlation to other modalities complete the documentation [9–12].
Concepts of Interpretation Based on Ultrasound BI-RADS Descriptors
The categorization of a mass found in all modalities relates to a 3D macropathological tissue lesion. The pathology defines lesion shape, margin, and texture. These features have already been described individually for the varying modalities. Uniform wording of the major diagnostic criteria for all modalities would be logical. The BI-RADS concept stepped first in this direction and was designed primarily as a mammographic language with a clear, defined terminology. In 2003, the ACR published the Breast Imaging Atlas, which is a BI-RADS lexicon for mammography, US, and MRI. The US chapters were originally arranged under the chair of Ellen B. Mendelson [11], and descriptors and diagnostic criteria are presented with increasing probability of malignancy. Descriptors of a mass include shape, orientation, margin, boundary, echo pattern, posterior acoustic features, and characteristics of surrounding tissue, as well as associated distinguishing findings. The combination of several descriptors predicts malignancy better than one single descriptor. However, the most relevant descriptors for characterizing a US lesion continue to comprehend shape, margin, and orientation taken together. The reader should use further explanatory elements indicated in the guidance chapters of the atlas, such as clinical context conditions, tumour biology, and epidemiological prevalence, to cover the complex field of breast lesions. Assumptions regarding the expected prevalence and individual risk for cancer in a patient drive the intuitive recommendation for or against a biopsy and influence the choice of a final BIRADS assessment category. In other words, the threshold for performing a biopsy is lower for a probably benign lesion compared with a screening setting if advanced patient age, large lesion, palpability, or individual highrisk situation are concerns to the reader. BI-RADS categories 3–5 imply a defined probability of malignancy for each category. For BI-RADS 3, these probabilities are <2%, for BI-RADS 4 between 3% and 94%, and for BIRADS 5⩾95%.
Most European US societies have adopted or modified the ACR BI-RADS US guidelines. In addition to the 2003 US descriptors, various features have been suggested, such as elastic compressibility, movability, 3D criteria, detailed lymph node morphology, and others. Further prospective multicenter studies still have to validate the complementary diagnostic importance of such associated features as an adjunct to the basic characteristics of a lesion [12, 13]. Several authors disclosed that interobserver agreement with the new BI-RADS terminology is good and validated the lexicon in retrospect following landmark studies in the 1990s. Only fair agreement exists in most studies for margin evaluation. Further, a trend toward lower concordance was noted for evaluating small masses. Classification into subdivisions 4a, 4b, and 4c was more or less reproducible. Despite limitations of the BI-RADS lexicon, most authors agree that stratification predicting the likelihood of malignancy could be useful for decision making and communication with patients and between researchers, physicians, and physicians of different specialties [14– 16]. The updated second edition of BI-RADS US guidelines [17] will reemphasize the importance of basic features, such as mass shape, margins, and orientation on one hand, and associated findings such as an adjunct on the other. The amended chapters cover expanded general issues, detailed lexicon images and US descriptors, reporting system, and guidance. Figure 3 presents a training schema for beginners in the field of breast diagnostics that can be used to learn standardized BI-RADS US reading of larger masses. This schema has no scientific proof for use in daily workup. Table 2 highlights some underlying intrinsic and extrinsic concepts of BI-RADS US assessment categories that must be considered in daily work.
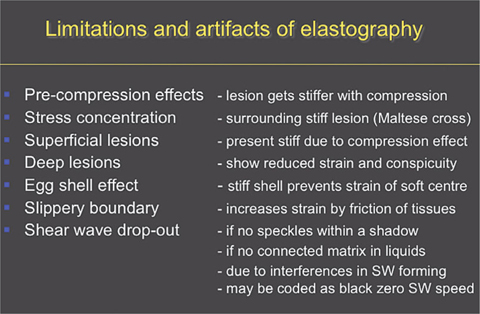

Fig. 3
Limitations and artefacts of elastography
Table 2
Underlying concepts of Breast Imaging and Reporting Data System ultrasound (BI-RADS US) assessment categories
– | Categorization and management depend on the most suspicious diagnostic criterion |
– | Benign lesions must look typically benign; no suspicious image descriptor |
– | Malignant lesions frequently show one or more suspicious criterion |
– | Predefined thresholds for positive predictive value or cancer risk influence classification in categories 2–5 |
– | Overall BI-RADS category must consider further clinical context conditions, expected prevalence, and other risk factors besides morphological criteria of each imaging modality assessment category |
– | Typical indicators of benignity, such as cysts, fat in a lesion (hamartoma, lipoma), or benign macrocalcification (popcorn calcification with fibroadenoma) diagnosed by multimodality evaluation can downgrade overall assessment category compared with US category |
– | Indicators of potential malignancy in other modalities or high-risk patients can upgrade overall assessment category compared with US category |
– | Overall assessment category should also be based on the most urgently needed procedure. This point of view ensures critical re-evaluation of final assessment category |
Concepts of Interpretation and Clinical Decision Making
The US characterization of a lesion in daily routine follows a reproducible diagnostic algorithm and should involve fundamental US and all advanced applications of the used US system, preferably on a one-click basis.
First, the reader must define whether or not the lesion resembles a typical benign finding, such as cyst, lipoma, lymph node, or previously know scar or fibroadenoma (Fig. 3). Complicated cysts with internal debris are challenging: when the debris is mobile or a fluid-debris level is seen, complicated cysts can be dismissed as benign findings, i.e., BI-RADS US category 2 [11, 18].
Second, a typical oval-shaped, hypoechoic lesion with circumscribed margins and horizontal orientation in young women is most likely a fibroadenoma (Fig. 4). Short-term follow-up can be used. Several studies concluded that short-term follow-up of such BI-RADS US category 3 lesions is associated with a cancer rate <2% [19–21]. Being >45 years, having palpability, or any preselection that enriched cancer cases in the collective, are associated with cancer rates <2%. In a recent study, 0.8% of 4,000 women with lesions that were initially classified as probably benign proved to be malignant at follow-up. The most frequent reason for a false-negative assessment on US was failure to recognize suspicious margin characteristics (28 of 32 malignancies; 87.5%). Malignancy was more frequent in palpable (2.4%; 21 of 859) than nonpalpable (0.4%; 11 of 3141) lesions [22]. As an isolated finding, homogeneous complicated cysts and clustered microcysts can be classified as probably benign, particularly if the lesion is new or rather small or deep, i.e., diagnostic uncertainty exists [18].
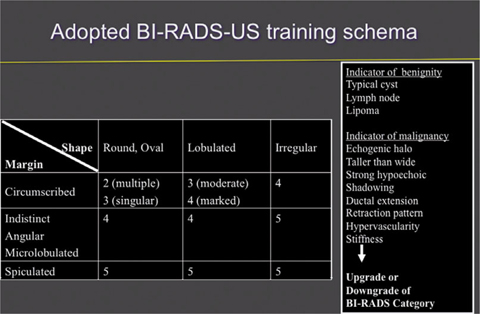

Fig. 4
Adopted Breast Imaging Reporting and Data System Ultrasound (BI-RADS US) training schema. BIRADS characterization of a mass can be taught using basic descriptors and associated findings that upgrade or downgrade overall assessment category. The teaching schema aims to support beginners in the field of breast diagnosis. This schema provides no scientific proof to be used in daily workup, as it can miss cancers
Third, detailed analysis of US morphology, vascularity, and elasticity of a lesion should disclose any suspicious basic descriptor or suspicious associated finding. The presence of suspicious descriptors results in a BIRADS US category 4 or 5, depending on the total number and character of these descriptors. A biopsy is recommended in these cases and also in benign-looking lesions that are significantly increasing in size during follow-up (Fig. 5) [11].
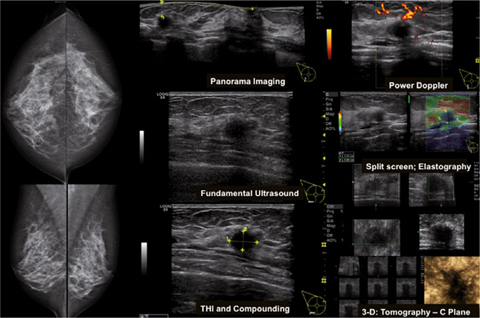

Fig. 5
A 53-year-old patient presenting with mammographic- negative and ultrasound (US)-positive invasive cancer. Histology is not otherwise specified (NOS), formerly named ductal invasive cancer and ductal carcinoma in situ (DCIS). No palpable finding. Diagnostic US criteria including elastography point to malignancy. American College of Radiology (ACR) density 2 (scattered fibroglandular tissue composition); Breast Imaging Reporting and Data System mammography (BI-RADS MG) 1; BI-RADS US 5. This lesion had been misinterpreted at another facility as a cyst in the presence of other fibrocystic findings. THI tissue harmonic imaging
Updated Role of Ultrasound
US studies in up to 12,000 asymptomatic patients yielded tumour detection rates of only 0.3–0.4%; however, a similar size and stage was reported compared with mammography- detected clinically occult cancers. The advantage of US as an adjunct to mammography is greatest in women with palpable lesions and those at high risk, including women with dense breasts, which is a risk factor. US signs of malignancy develop with increasing tumour size. No single diagnostic sign can pick up all cancers due to their heterogeneous appearance. Patients with a high mammographic density (<75%) present in meta-analyses with fourfold increased risk compared with women with low-radiodense breasts and a twofold increased risk compared with women with scattered fibroglandular breasts [7]. The sensitivity of standard US for breast cancer is 55– 95%. US transfers an additional diagnostic yield of 30– 40% in comparison with mammography to patients with radiodense breasts in the incidence setting (Fig. 5). The updated American College of Radiology Imaging Network (ACRIN) follow-up study focuses on cancer detection in patients at increased risk due to radiodense breasts and those under surveillance after breast cancer or other conditions. Of 111 imaging-detected cancers, 33 cancers were found by mammography only, 32 by US only, 26 by both methods, and nine by MRI only [23]. In national screening programs, mammography is still the method of choice of early breast cancer detection. The upcoming Austrian national screening program will add US examination for all women presenting with an ACR density level 3 and 4 (dense and extremely dense).
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree








