, Carmen L. Menendez2, Rodolfo Montironi3 and Liang Cheng4
(1)
Department of Surgery and Pathology, University of Cordoba Faculty of Medicine, Cordoba, Spain
(2)
Pathology Department, Hospital de Cabueñes, Gijón, Spain
(3)
Department of Biomedical Sciences and Public Health, Polytechnic University of the Marche Region (Ancona), Torrette, Italy
(4)
Department of Pathology, Indiana University School of Medicine, Indianapolis, Indiana, USA
2.1 Introduction
2.1.1 Basic Anatomy and Histology
Urinary bladder
The bladder is a hollow muscular organ whose main function is that of a reservoir. When empty, the adult bladder lies behind the symphysis pubis, and is largely a pelvic organ. When full, the bladder rises above the symphysis and can readily be palpated.
The empty bladder has an apex (superior surface), two infralateral or anterolateral surfaces, a base (posterior surface), and a neck. The apex extends a short distance above the pubic bone and ends as a fibrous cord derivative of the urachus. This fibrous cord extends from the apex of the bladder to the umbilicus between the peritoneum and the transversalis fascia.
The apex of the bladder is apposed to the uterus and ileum in the female, and the ileum and pelvic portion of the colon in the male.
The base of the bladder faces posteriorly and is separated from the rectum by the uterus and vagina in the female, and by the vasa deferentia, seminal vesicles, and ureters in the male.
The anterolateral surface on each side of the bladder is apposed to the pubic bone, levator ani, and obturator internus muscles, but the central anterior bladder is separated from the pubic bone by the retropubic space, that contains abundant fat and venous plexuses.
The neck of the bladder, its most inferior part, connects with the urethra. When the bladder is distended with urine, the neck remains fixed and stationary, whereas the dome rises above the pelvic cavity into the lower abdomen, touching the posterior aspect of the lower anterior abdominal wall and the small and large bowel.
Beneath the urothelial lining of the inner bladder, there is loose connective tissue that permits considerable stretching of the mucosa. As a result, the urothelial mucosal lining is wrinkled when the bladder is empty but smooth and flat when distended.
This arrangement exists throughout the bladder except at the trigone, where the mucous membrane adheres firmly to the underlying muscle; consequently, the trigone is always smooth, regardless of the level of distension.
The bladder is supplied by the superior, middle, and inferior vesical arteries, all of which are branches of the anterior division of the hypogastric artery.
Between the bladder wall proper and the outer adventitial layer, there is a rich plexus of veins that ultimately terminate in the hypogastric veins after converging in several main trunks.
The bladder lymphatics drain into the external iliac, hypogastric, and common iliac lymph nodes. There are rich lymphatic anastomoses between the pelvic and genital organs.
The bladder is richly innervated by divisions of the autonomic nervous system. Sympathetic nerves originate from the lower thoracic and upper lumbar segments, mainly T11-T12 and L1-L2.
These sympathetic fibers descend into the sympathetic trunk and the lumbar splanchnic nerves, connecting with the superior hypogastric plexus, an inferior extension of the aortic plexus.
Parasympathetic nerves arise from sacral segments S2-S4, and these form the rich pelvic parasympathetic plexus. This plexus joins the sympathetic hypogastric plexus, and vesical branches emerge from this plexus toward the bladder base, innervating the bladder and urethra.
Paraganglia are rarely found in routine sections of the urinary bladder. Paraganglionic tissue typically demonstrates immunoreactivity with Neuroendocrine markers such as chromogranin, synaptophysin, and neuron specific enolase. The sustentacular cells exhibit immunostaining for S-100 protein.
The urothelium is a unique stratified epithelium of variable thickness. The number of cell layers depends on the degree of distension of the bladder, usually varying from three to seven layers. When distended, the bladder is three to six cell layers thick, although the typical biopsy contains about five layers; in the contracted state, it consists of six to eight layers (Figs. 2.1, 2.2, and 2.3)
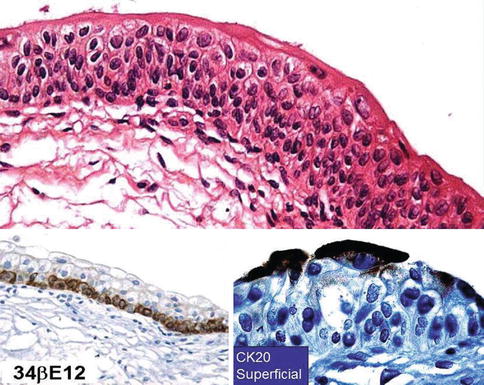
Fig. 2.1
Microscopic appearance of normal uothelium (upper). 34BE12 highlights basal cell layer (lower left) and CK 20 highlights superficial cells (lower right)
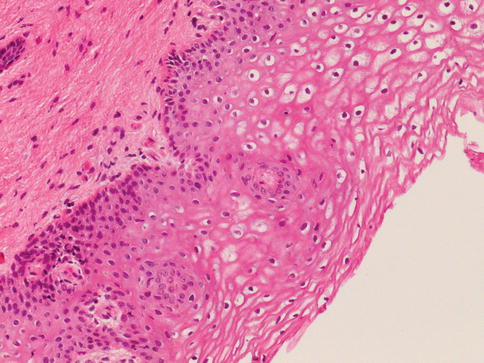
Fig. 2.2
Non-keratinized squamous epithelium in the trigone
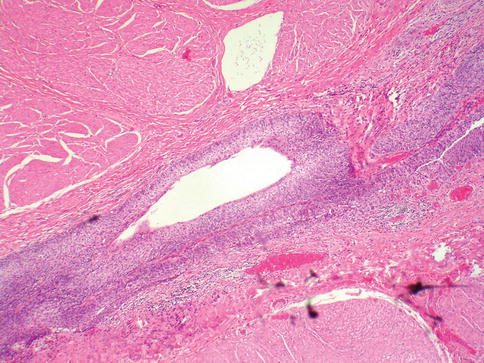
Fig. 2.3
Urothelial lined urachus
The normal urothelium contains a layer of large superficial (umbrella cells) cells that are frequently multinucleated. These cells have abundant eosinophilic cytoplasm, with large nuclei whose long axes are perpendicular to those of the smaller cells of the underlying basal and intermediate cell layers. Superficial umbrella cells express uroplakins and CK 20 on immunohistochemistry.
Basal and intermediate cells are located between the basal lamina and the superficial cells. These cells are morphologically identical to each other, and are distinguished only by their position in the mucosa. They are regularly arranged, with distinct cell boundaries and oval, round, or fusiform nuclei with occasional prominent nuclear grooves.
The basal layer of epithelial cells expresses Bcl-2 while the intermediate cells express RB1 and PTEN at varying intensities. HER2/neu and p53 are not expressed by normal urothelial cells. Ki67, indicating proliferation, may not be expressed in a single field.
The long axis of the basal and intermediate cells is perpendicular to the basement membrane. Basement membrane markers such as laminin and type IV collagen may be useful diagnostically in select cases to define the basement membrane, but are not routinely employed.
The lamina propria, located beneath the basement membrane, consists of a compact layer of fibrovascular connective tissue. It contains an incomplete muscularis mucosa composed of thin delicate smooth muscle fibers that may be mistaken for muscularis propria in biopsy specimens.
In biopsy specimens, these smooth muscle fibers may appear as a continuous layer, a discontinuous or interrupted layer, or as scattered thin bundles of smooth muscle fibers that do not form an obvious layer. The muscle fibers lie parallel to the mucosal surface, midway between the epithelium and the underlying muscularis propria (Figs. 2.4 and 2.5)
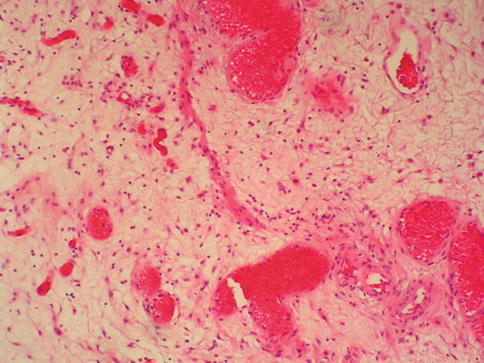
Fig. 2.4
Incomplete muscularis mucosae
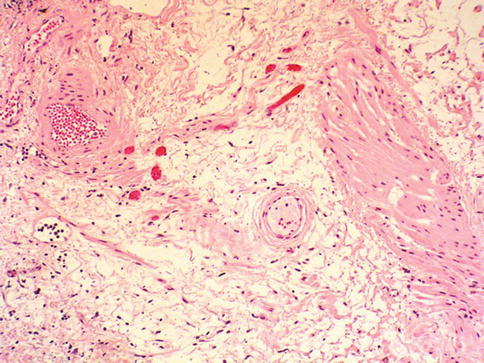
Fig. 2.5
Occasionally, smooth muscle fibers of muscularis mucosae have an hypertrophic appearance
Moderate size or large thick-walled blood vessels are a constant feature of the lamina propria, running parallel to the surface urothelium in close association with the smooth muscle fibers of the muscularis mucosa.
The muscle proper of the bladder, the muscularis propria, is moderately thick and consists of an inner longitudinal layer, middle circular layer, and outer longitudinal layer. It spirals around each ureteral orifice and increases in thickness around the internal urethral orifice, forming the internal sphincter of the bladder. The muscularis is surrounded by a coat of fibroelastic connective tissue, the adventitia, and perivesical fat.
The Urachus
The urachus is an intra-abdominal embryonic remnant. It contains the allantois, connecting the apex of the urinary bladder to the body wall at the umbilicus.
At birth, the dome of the bladder and the umbilicus are closely apposed, and the urachus is only 2.5–3 mm long, with a diameter of 1 mm throughout most of its course and 3 mm where it joins the bladder.
The urachus lies in a space anterior to the peritoneum, bounded anteriorly and posteriorly by the umbilicovesical fascia. Laterally, it is bounded by the two umbilical arteries that, in turn, are surrounded by umbilicovesical fascia.
Inferiorly, the umbilicovesical fascial layers cover the surface of the dome of the bladder. This space, the space of Retzius, is roughly pyramidal, and fascial planes separate it from the peritoneum and other structures. At the junction with the urinary bladder, the adult urachus is 4–8 mm wide, narrowing to about 2 mm at its superior end.
The urachus has three segments, including the supravesical, intramural, and intramucosal segments. Tubular urachal remnants are found within the wall of the urinary bladder in approximately one-third of adults and are evenly distributed between men and women.
There are three architectural patterns of intramural urachal canals varying from simple tubular canals to complex branching canals.
The mucosal portion of the urachus may have a wide diverticular opening, papilla, or a small opening flush with the mucosal surface. The majority (70 %) of intramural urachal remnants are lined by urothelium; the remainder are lined by columnar epithelium, occasionally with small papillae or, rarely, mucous goblet cells or mucus-secreting columnar epithelium.
The Renal Pelvis and Ureters
The lumen of the renal pelvis and ureter is lined by urothelium that rests on a basement membrane. The urothelium is composed of 3–5 layers of cells in the pelvis and 4–7 layers of cells in the ureter.
The pelvis and ureter have a continuous muscular wall that originates in the fornices of the minor calyces as small interlacing fascicles of the smooth muscle cells. The muscularis propria is not divided into distinct layers.
The Urethra
The epithelium of the urethra is derived from the urogenital sinus. In females, the epithelium of the urethra is derived from endoderm of the urogenital sinus, while the surrounding connective tissue and smooth muscle arise from splanchnic mesenchyme.
In males the epithelium also is derived from the urogenital sinus except in the fossa navicularis, where it is derived from ectodermal cells migrating from the glans penis. As in females, the connective tissue and smooth muscle surrounding the male urethra is derived from splanchnic mesenchyme.
The male urethra is 15–20 cm long and is divided in three anatomical segments. The prostatic urethra begins at the internal urethral orifice at the bladder neck and extends through the prostate to the prostatic apex. In the central part of the urethral crest is an eminence called the verumontanum.
The verumontanum contains a slitlike opening that leads to an epithelium-lined sac called the prostatic utricle, a Mullerian vestige. The ejaculatory ducts empty into the urethra on either side of the prostatic utricle. The membranous urethra extends from the prostatic apex to the bulb of penis.
Cowper’s glands are located on the left and right sides of the membranous urethra and their ducts empty into it. Bulbourethral glands are located in the proximal (bulbous) portion of the penile urethra. In addition, scattered mucus-secreting periurethral glands (Littre’s glands) are present at the periphery of the penile urethra except anteriorly.
The female urethra is approximately 4 cm long, and, at its periphery, contains paraurethral Skene’s glands.
The type of epithelium lining the urethra varies along its length. In general, urothelium lines the prostatic urethra, pseudostratified columnar epithelium lines the membranous segment and most of the penile urethra, and nonkeratinized stratified squamous epithelium lines the fossa navicularis and external urethral orifice.
In females, the proximal one-third of the urethra is lined by urothelium and the distal two-thirds by nonkeratinized stratified squamous epithelium.
The urothelium has a characteristic immunophenotype. It expresses cytokeratins of both low and high molecular weights, including keratins 7, 8, 13, and 19; cytockeratin 18 and 20 are present in the superficial cells.
This pattern of expression differs from that of normal stratified squamous epithelium, that shows predominantly high molecular weight keratin immunoreactivity, and from endometrium, endocervix, colorectum, and prostate, that demonstrate a preponderance of low molecular weight keratin.
High molecular weight keratin immunoreactivity is restricted to the basal cell layer of the urothelium and squamous mucosa of the trigone.
2.2 Urinary Bladder
2.2.1 Urothelial Carcinoma
Overview
Carcinoma of the urinary bladder is the fourth most common malignancy in men, and more than 90 % of bladder cancer cases are urothelial (transitional cell) carcinoma, whereas primary squamous cell carcinoma, adenocarcinoma, small cell carcinoma, and other tumors are less common.
Bladder cancer is morphologically heterogeneous disease. The classification of urinary bladder neoplasia used in this chapter follows the most recent World Health Organization (WHO) classification of tumors of the urinary system.
2.2.2 Flat Intraepithelial Lesions
The classification of nonpapillary (flat) intraepithelial lesions and conditions of the urothelium has evolved over the years (Table 2.1). This classification includes epithelial abnormalities (reactive urothelial atypia and flat urothelial hyperplasia), presumed preneoplastic lesions and conditions (keratinizing squamous and glandular metaplasia, and malignancy associated cellular changes) as well as preneoplastic (dysplasia) and neoplastic noninvasive (carcinoma in situ) lesions (Figs. 2.6, 2.7, 2.8, 2.9, 2.10, 2.11, 2.12, 2.13, 2.14, 2.15, 2.16, 2.17, 2.18, 2.19, and 2.20)
Table 2.1
Classification of flat urothelial lesions of the urinary bladder based on the Ancona International Consultation
Flat urothelial hyperplasia
Reactive urothelial atypia
Presumed preneoplastic lesions and conditions
Keratinizing squamous metaplasia
Intestinal metaplasia
Malignancy associated cellular changes
Preneoplastic lesions
Dysplasia
Neoplastic non-invasive lesion
Urothelial carcinoma in situ
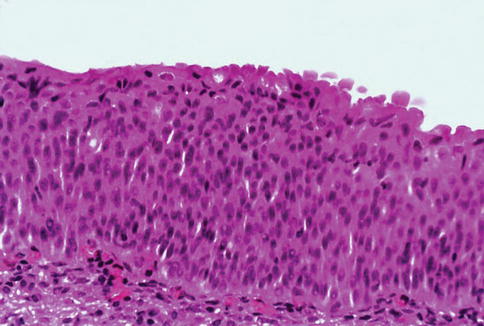
Fig. 2.6
Microscopic appearance of flat urothelial hyperplasia
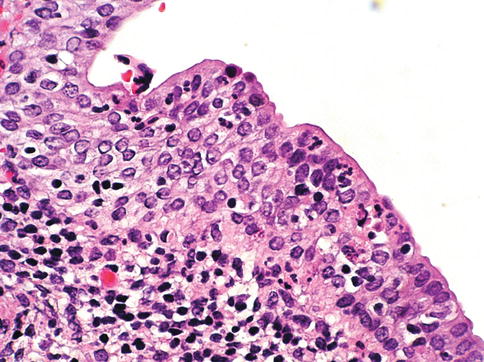
Fig. 2.7
Microscopic changes of reactive urothelium
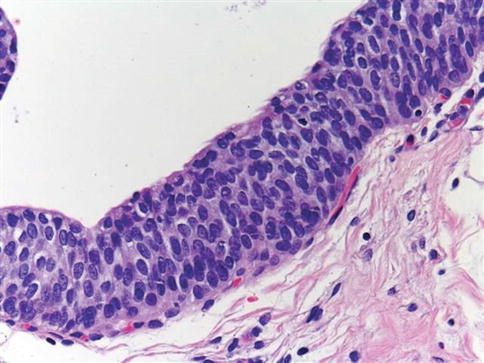
Fig. 2.8
Microscopic appearance of urothelial dysplasia
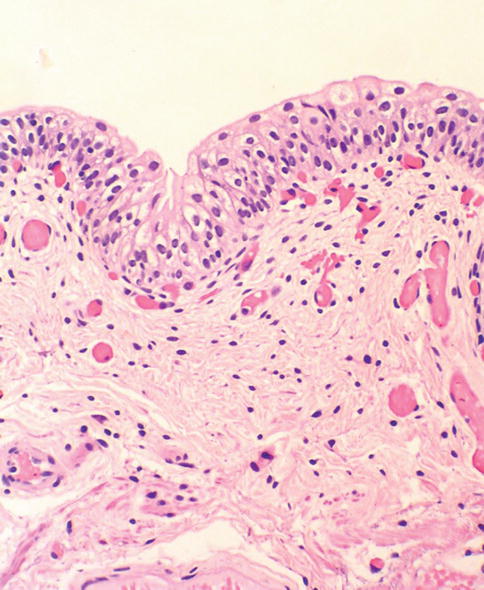
Fig. 2.9
A comparison with normal urothelium is helpful in diagnosing flat urothelial lesions
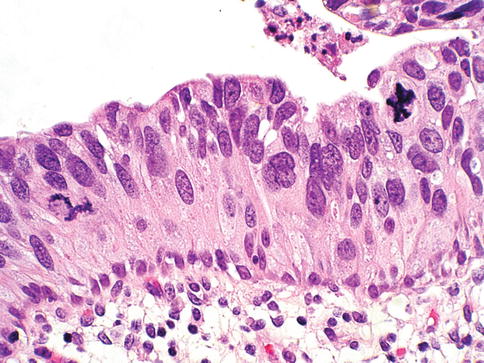
Fig. 2.10
Urothelial carcinoma in situ
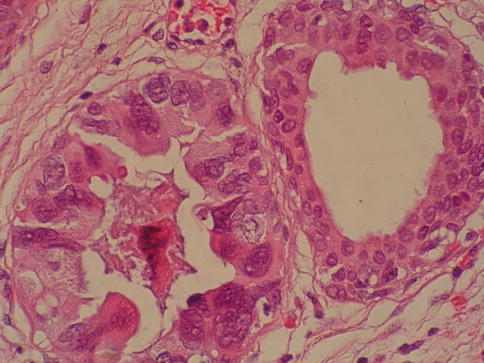
Fig. 2.11
Urothelial carcinoma in situ in von Brunn nest showing marked pleomorphism
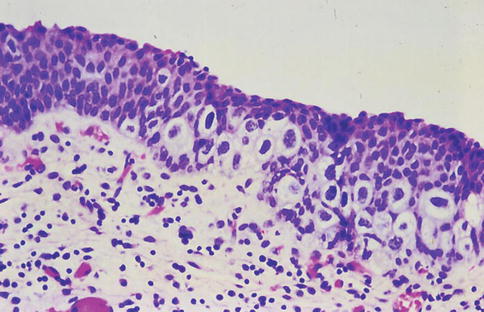
Fig. 2.12
Pagetoid urothelial carcinoma in situ
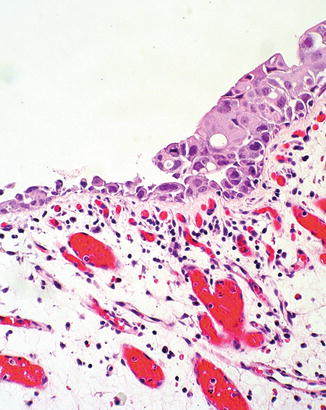
Fig. 2.13
Urothelial carcinoma in situ with squamous features (right)
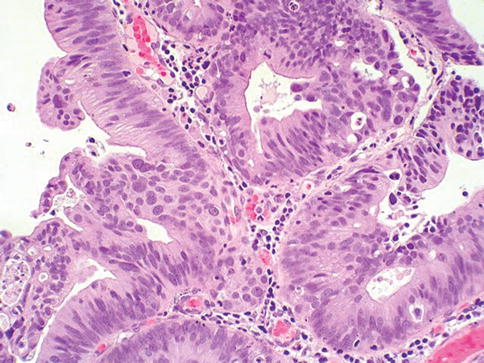
Fig. 2.14
Urothelial carcinoma in situ with glandular differentiation
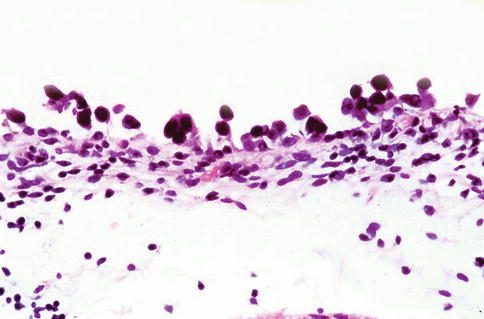
Fig. 2.15
Urothelial carcinoma in situ, clinging type
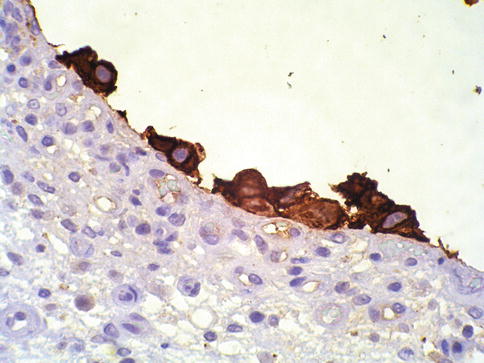
Fig. 2.16
Urothelial carcinoma in situ, clinging type. Keratin staining highlights residual cells
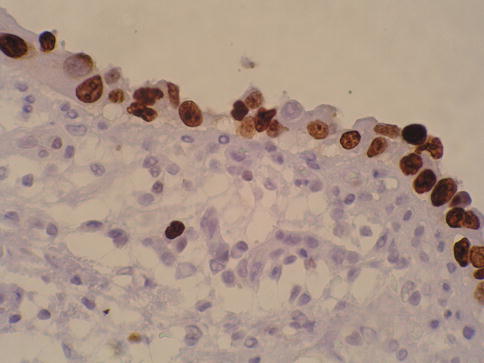
Fig. 2.17
P53 nuclear accumulation in urothelial carcinoma in situ
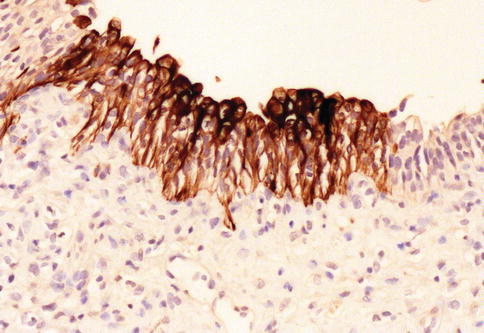
Fig. 2.18
Aberrant CK20 expression in urothelial carcinoma in situ
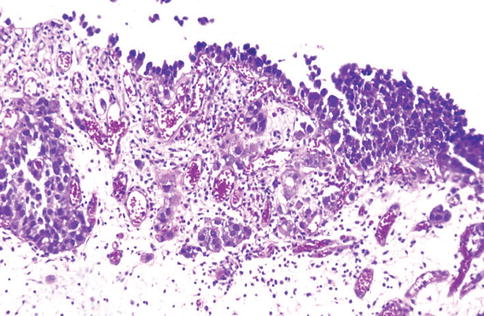
Fig. 2.19
Urothelial carcinoma in situ with microinvasion
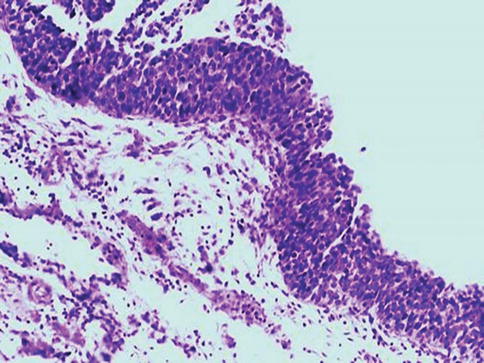
Fig. 2.20
Small cell type of urothelial carcinoma in situ
Recent studies have shown that 50 % of the histologically normal urothelium adjacent to superficial urothelial carcinoma harbors genetic anomalies on chromosome 9, similar to the anomalies found in the coexisting carcinoma. In addition, non-diploid nuclear DNA histograms occur in 4–54 % of histological normal urothelium adjacent to bladder tumors. These genetic alterations suggest a neoplastic potential for flat urothelial lesions, regardless of whether cytologic atypia is present or not.
Flat urothelial hyperplasia (simple hyperplasia)
Urothelial hyperplasia is characterized by markedly thickened mucosa with an increase in the number of cell layers, usually 10 or more. However, it is not necessary to count the number of cell layers for the diagnosis (Table 2.2). The cells in urothelial hyperplasia do not show any significant cytologic abnormalities, although slight nuclear enlargement may be focally present.
Table 2.2
Comparison of flat intraepithelial lesions
Reactive atypia
Hyperplasia
Dysplasia
Carcinoma in situ
Cell layers
Variable
>7 cells
Variable
Variable
Polarization
Slightly abnormal
Normal
Slightly abnormal
Abnormal
Cytoplasm
Vacuolated
Homogeneous
Homogeneous
Homogeneous
N:C ratio
Normal or slightly increased
Normal or slightly increased
Slightly increased
Increased
Nuclei
Anisonucleosis
Normal
Normal
Mild
Moderate to severe
Borders
Regular/smooth
Regular/smooth
Notches/creases
Pleomorphic
Chromatin
Fine/dusty
Fine
Slight hyperchromasia
Coarse/hyperchromatic
Chromatin distribution
Even
Even
Even
Uneven
Nucleoli
Large
Small/absent
Small/absent
Large/prominent
Mitotic figures
Variable
Absent
Rare and rare
Often
Denudation
Variable
No
No
Variable
Cytokeratin 20
Surface
Surface
Variable
Variable
Stromal microvascular proliferation
Variable
Variable
Less prominent
Often prominent
Morphologic evidence of maturation from base to surface is generally evident. Urothelial compression artifact or tangential sectioning of mucosa with pseudopapillary growth (lacking a true vascular core) may resemble flat urothelial hyperplasia.
Flat urothelial hyperplasia has been observed in association with a variety of conditions including inflammatory disorders, urolithiasis, papillary urothelial hyperplasia, dysplasia, carcinoma in situ and low grade papillary tumors. Flat urothelial hyperplasia has been considered by some authors to be the source of low grade papillary neoplasia.
When seen as an isolated phenomenon, there is no evidence to suggest that primary urothelial hyperplasia has a pre-malignant potential. Likewise, molecular analyses showing chromosome 9q deletions and mutations in the Fibroblast Growth Factor Receptor 3 (FGFR3) gene in both urothelial hyperplasia and low grade papillary neoplasia suggest that this lesion may be clonally related to the papillary tumors in bladder cancer patients.
Urothelial reactive atypia
Urothelial abnormalities whose architectural and cytologic changes are of lesser degree than those of dysplasia have often been termed atypia. The term atypia is, by its very nature, non-specific. Two similar categories of atypia have been recently recognized, namely reactive atypia and atypia of unknown significance. Both of them are placed among the “benign” urothelial abnormalities.
Reactive atypia is characterized by mild nuclear abnormalities occurring in acutely or chronically inflamed urothelium. In most cases, there is a history of cystitis, instrumentation, infection, stones, or previous therapy. The epithelium may or may not be thickened in reactive atypia. The cells are often larger than normal, with more abundant cytoplasm (squamoid appearance) than normal urothelial cells.
Nuclei are uniformly enlarged, vesicular, and may have prominent centrally located nucleoli. Mitotic figures may be frequent but occur in the lower epithelial layers. Inflammatory cells occupying the lamina propria and infiltrating into the urothelium are present. CD44 is positive in reactive atypia, and CK20 or p53 are negative.
The term “atypia of unknown significance” was introduced to describe lesions in which the pathologist was uncertain whether the changes were reactive or preneoplastic (dysplasia). Atypia of unknown significance is characterized by nuclear changes similar to those seen in reactive atypia. However, the degree of nuclear pleomorphism and hyperchromasia is greater than in reactive atypia and dysplasia cannot be definitely ruled out.
Inflammation in the lamina propria with urothelial infiltration is often present. However, the cellular changes seem to be disproportionate to the degree of inflammation.
The clinical outcome of patients with atypia of unknown significance is identical to that of patients with reactive atypia. The use of the designation “atypia of unknown significance” is discouraged.
Urothelial dysplasia (low grade intraurothelial neoplasia)
Urothelial dysplasia is defined as abnormal urothelium with cytologic and architectural changes that do not meet all the criteria for an unequivocal diagnosis of urothelial carcinoma in situ. The overall appearance is that of the urothelium in low grade papillary urothelial carcinoma (Table 2.2).
The cytologic abnormalities in urothelial dysplasia, characterized by cellular crowding, loss of orderly maturation, and loss of cellular polarity, are not present in the full thickness of the urothelium. Occasionally, there may be an increased number of cell layers. The superficial umbrella cells are usually present. Most cellular abnormalities in dysplasia are restricted to the basal and intermediate cell layers.
Individual dysplastic cells show enlarged nuclei and nucleoli with irregular contours and coarsening of the chromatin. Multiple nucleoli and nuclear overlapping may be seen. The cells often show cytoplasmic clearing. Mitotic figures, when present, are generally basally located. The transition from normal to abnormal urothelium is subtle Non-dysplastic urothelial cells are often dispersed among the dysplastic cells.
Nuclear and architectural features are the primary criteria for distinguishing dysplasia from reactive atypia and urothelial carcinoma in situ.
Grading of urothelial dysplasia and the use of the term “atypia” as a synonym for urothelial dysplasia is discouraged.
Primary dysplasia occurs in the absence of urothelial tumors. In one autopsy study, primary dysplasia was present in up to 6.8 % of males and 5.7 % of females. These patients are predominantly middle-aged men with irritative symptoms with or without hematuria. Dysplasia is not cystoscopically visible. It is estimated that de novo (primary) dysplasia progresses to bladder neoplasia in 14–19 % of cases.
Secondary dysplasia is seen in patients with a history of bladder neoplasia. The incidence of dysplasia in patients with established bladder neoplasia varies from 22 to 86 % and approaches 100 % in patients with invasive carcinoma. The presence of urothelial dysplasia indicates urothelial instability. Recurrence rate was 73 % of patients with superficial neoplasia and concomitant dysplasia compared with 43 % without coexisting dysplasia.
Immunohistochemistry
CK20 immunostaining is limited to the superficial cell layers in normal urothelium; in contrast, CK20 immunostaining is usually present in the superficial and intermediate cell layers of dysplastic urothelium (aberrant CK20 exoression). Positive CD44 immunostaining is observed only in the basal cells in normal urothelium, and is either absent entirely or present only in scattered cells in urothelial dysplasia, whereas full-thickness positive membranous CD44 staining is typical of reactive urothelium (Table 2.3).
Table 2.3
Immunohistochemical features of flat-related lesions
Normal
Flat urothelialhyperplasia
Reactive Atypia
Atypia of unknownsignificance
Dysplasia
CK20
Limited toumbrella cells
Limited toumbrella cells
Limited toumbrella cells
Limited toumbrella cells
Deep layers
CD44
Limited tobasal cells
Limited tobasal cells
Increased reactivityin all cell layers
Increased reactivityin all cell layers
Absent
p53
Absent
Absent
Absent
Absent
Positive
Dysplastic cells show increased p53 expression, whereas p53 nuclear accumulation is predominantly undetectable or only weakly evident in the basal and parabasal cells in reactive urothelium. P16 has been reported variable positive in urothelial dysplasia and weak/patchy in reactive urothelium.
Urothelial carcinoma in situ (high grade intraurothelial neoplasia)
Urothelial carcinoma in situ is a flat non-invasive lesion in which the urothelium is entirely composed of cytologically malignant cells. The patient’s age at diagnosis ranged from 32 to 90 years (mean, 66 years). The male-to-female ratio in patients with carcinoma in situ is approximately 7:1.
Clinical presentations include gross and microscopic hematuria, irritative symptoms (dysuria, pain, and frequency), nocturia, and sterile pyuria. Approximately 25 % of patients are asymptomatic. Carcinoma in situ usually is multifocal, with a predilection for the trigone, lateral wall, and dome of the bladder. Cystoscopically, it may appear as erythematous velvety or granular patches, although it may also be visually undetectable. Erythematous changes are often apparent at gross examination.
De novo or isolated carcinoma in situ (often referred to as primary carcinoma in situ) accounts for 1–3 % of bladder neoplasms. Mapping studies of cystectomy specimens show involvement of the prostatic urethra and of the ureter in as many as 67 and 57 % of cases, respectively.
Urothelial carcinoma in situ has a high likelihood of progressing to invasive carcinoma if left untreated. The mean interval between a diagnosis of carcinoma in situ and the detection of cancer progression is 5 years. The actuarial progression-free survival, cancer-specific survival, and all-cause survival rates are 63, 79, and 55 %, respectively, at 10 years.
Factors predictive of progression include multifocality, coexistent bladder neoplasia, DNA aneuploidy, CCND3 gene amplification, prostatic urethral involvement, and recurrence after treatment. Urothelia carcinoma in situ is often associated with invasive carcinoma elsewhere in the bladder (referred as secondary carcinoma in situ).
Microscopically, urothelial carcinoma in situ is characterized by flat, disordered proliferation of urothelial cells with marked cytologic abnormalities. The morphological diagnosis of carcinoma in situ requires severe cytological atypia (anaplasia).
Full thickness-change is not required, and it ranges from one cell layer to the thickness of hyperplasia (greater than 7 cells). Superficial (umbrella) cells may or may not be present. Marked disorganization of cells is characteristic, with loss of cellular polarity and decreased cellular cohesiveness.
The tumor cells tend to be large and pleomorphic, with moderate to abundant cytoplasm. Nevertheless, the cells of carcinoma in situ are sometimes small with a high nucleus to cytoplasmic ratio. The chromatin tends to be coarse and clumped. Nucleoli may be multiple and they are large and prominent in at least some of the cells. Mitotic figures, which are often atypical, are seen in the uppermost layers of the urothelium.
Tissue edema, vascular ectasia and proliferation of small capillaries are frequently observed in the lamina propria.
Immunohistochemistry
Carcinoma in situ shows intense CK 20 and p53 positivity in the majority of malignant cells. Increased Ki-67 labeling is noted in carcinoma in situ but this can be seen also in reactive atypia of the urothelium thus limiting its usefulness in practice. P16 is typically positive in carcinoma in situ (about 90 % of cases) and Racemase is positive in 50–80 % of cases of carcinoma in situ.
The neoplastic cells are uniformly negative for CD44 immunostaining. In contrast, cytokeratin 20 stainings show patchy cytoplasmic immunoreactivity only in the superficial umbrella cell layer and CD44 stains only the basal cells in the adjacent normal or reactive urothelium. Reactive urothelium is negative for p16 and Racemase, findings that need to be validated in larger cohorts.
Other markers of potential interest include HER-2neu diffuse expression in carcinoma in situ with few positive cells in reactive urothelium.
Histological variants of urothelial carcinoma in situ
Awareness of the histologic diversity of carcinoma in situ may aid in the diagnosis of this important lesion. In practice, it is not necessary to mention these specific growth patterns or morphologic variants in the surgical pathology report (Table 2.4)
Table 2.4
Morphologic patterns of urothelial carcinoma in situ (CIS)
Large cell CIS
Small cell CIS
Denuding and “clinging pattern” of CIS
Pagetoid and undermining (lepedic) of CIS
CIS with squamous or glandular differentiation
CIS with microinvasion (CISmic)
Large cell carcinoma in situ
Large cell carcinoma in situ constitutes the most common morphologic form of this entity. Cytologic findings include nuclear pleomorphism, variably abundant cytoplasm and anaplastic nuclear features. In rare cases, large cell carcinoma in situ may have minor nuclear pleomorphism but still exhibits architectural disarray. Rarely, it may focally exhibit high pleomorphism with bizarre giant cells (pleomorphic/giant cell carcinoma in situ).
Small cell carcinoma in situ
The small cell pattern refers to the size of the cells and may or may not coexist with small cell carcinoma and is unrelated to neuroendocrine differentiation. In such cases, the pleomorphism is minimal, the cytoplasm is scant and nuclei are enlarged and hyperchromatic, with coarse unevenly distributed chromatin.
The scattered prominent nucleoli are distorted and angulated. Recognition of the small cell pattern of carcinoma in situ is important to avoid misdiagnosis of basal cell hyperplasia which has been observed in patients treated with bacille Calmette-Guérin (BCG). This shows a small cell pattern but lack nuclear atypia or loss of polarity.
Denuding and clinging carcinoma in situ
In some cases, the neoplastic urothelial cells are strikingly dyscohesive and undergo extensive exfoliation, with the result that biopsies may show only a few residual carcinoma cells on the surface (“clinging carcinoma in situ”) or no recognizable epithelial cells on the surface, a condition referred to as “denuding cystitis”.
In the clinging pattern of carcinoma in situ there is a patchy, usually single layer of atypical cells. In mucosal biopsies entirely lacking surface epithelium, carcinoma in situ may be present only in von Brunn’s nests. A careful search for carcinoma in situ in deeper sections or in other submitted biopsy fragments is important, and a recommendation for evaluation of urine cytology for carcinoma cells is warranted.
Pagetoid carcinoma in situ
Cancerization of the urothelium, shows either pagetoid spread (clusters or isolated single cells) or undermining or overriding of the normal urothelium (lepidic growth). Carcinoma in situ exhibiting pagetoid growth is characterized by large single cells or small clusters of cells within otherwise normal urothelium of ureter, urethra, prostatic ducts or in areas of squamous metaplasia. Individual cells showing pagetoid spread have enlarged nuclei with coarse chromatin; frequently, the cytoplasm is clear.
Pagetoid growth patterns can be found in up to 15 % of carcinoma in situ cases. Most patients are male and their ages range from 31 years to 78 years (mean, 64 years). Pagetoid carcinoma in situ is usually a focal lesion and is easily overlooked; it occurs in a clinical and histological setting of conventional carcinoma in situ with coexisting invasive urothelial carcinoma, and such patients essentially have the same progression and survival rates as patients without pagetoid changes.
In cases with extensive urothelial denudation, pagetoid carcinoma in situ may be focally present in adjacent otherwise normal-looking urothelium, thus alerting the pathologist to search for additional carcinoma in situ elsewhere in the bladder.
Since primary extramammary Paget’s disease of the external genitalia and of the anal canal may extend to the bladder, and conversely, some cases of pagetoid carcinoma in situ of the bladder may extend to the urethra, ureter, and external genitalia, differentiating between these two entities represents an important diagnostic and therapeutic challenge.
A panel of immunostains including cytokeratin 7, cytokeratin 20, and thrombomodulin may assist in differentiating pagetoid urothelial carcinoma in situ from extramammary Paget’s disease, which is known to be cytokeratin 7 positive and cytokeratin 20 negative.
Carcinoma in situ with squamous or glandular differentiation.
Rare cases of carcinoma in situ may exhibit squamous differentiation characterized by intercellular bridges. Carcinoma in situ with squamous features is most often observed in association with urothelial carcinoma showing extensive squamous differentiation elsewhere in the bladder.
A much less frequently encountered pattern is carcinoma in situ with morphological and immunohistochemical evidence of glandular differentiation. Some authors refer to this as adenocarcinoma in situ; such lesions may show papillary, cribriform, or flat morphology. Carcinoma in situ involving von Brunn’s nests, cystitis glandularis or cystitis cystica may be difficult to distinguish from adenocarcinoma in situ in the absence of concurrent invasive adenocarcinoma.
A recent report based on 25 cases of urothelial carcinoma in situ with glandular differentiation showed high ki-67 index and p53 accumulation, high nuclear and cytoplasmic p16 expression and diffuse PTEN expression, a phenotype that also characterized concurrent conventional carcinoma in situ. MUC5A, MUC2, CK20 and c-erbB2 were positive in all 25 cases of urothelial carcinoma in situ with glandular differentiation and CDX-2 was present in 19 cases; MUC1, CK7 or 34ßE12 was focally present in 21, 19 and 18 cases, respectively.
The authors concluded that urothelial carcinoma in situ with glandular differentiation is a variant of carcinoma in situ that follows the natural history of conventional urothelial carcinoma in situ. The immunophenotype suggests urothelial origin with the expression of MUC5A and CDX2 as signature for glandular differentiation.
Carcinoma in situ with microinvasion
Carcinoma in situ with microinvasion was initially defined as invasion into the lamina propria to a depth of 5 mm or less from the basement membrane. Recent consensus conference suggested that cases with more than 20 cells measured from the stromal-epithelial interface should be classified as fully invasive. Microinvasion appears as direct extension in cords (tentacular), single cells, or single cells and clusters of cells.
The clusters of cells may have retraction artifact that mimics vascular invasion. Stromal response may be present, but in most cases is absent. In cases with a prominent stromal inflammatory response the invasive neoplastic cells may be interspersed among lymphocytes, making them inconspicuous. In these circumstances, immunohistochemical staining with antibodies against cytokeratins (such as AE1/AE3) exposes the invading cells.
A potential pitfall to be avoided is the known positivity of myofibroblastas present in bladder’s lamina propria with antibodys against keratins.
Therapy-induced changes in the urothelium and mimics of urothelial flat neoplasia
Antineoplastic agents used in the bladder or systemically, such as thiotepa (triethylenethiophosphoramide), mitomycin C, cyclophosphamide, bacille Calmette-Guérin (BCG), and radiation therapy produce urothelial changes that can mimic cancer histologically (Figs. 2.21, 2.22, 2.23, and 2.24)
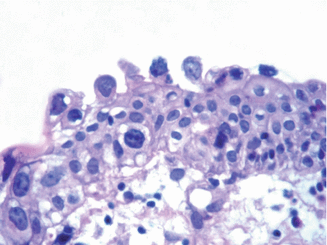
Fig. 2.21
Reactive urothelial atypia following mitomycin C therapy
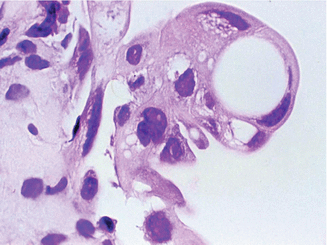
Fig. 2.22
Reactive urothelial atypia following thiotepa therapy
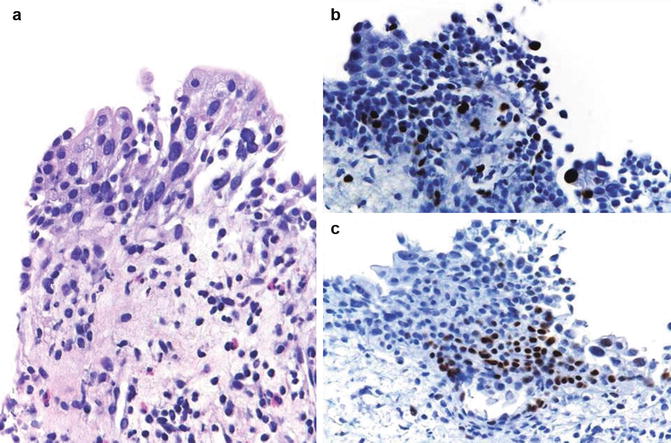
Fig. 2.23
Reactive urothelial atypia following cyclophosphamide therapy (a), showing low ki67 proliferation (b), and low p53 nuclear accumulation (c)
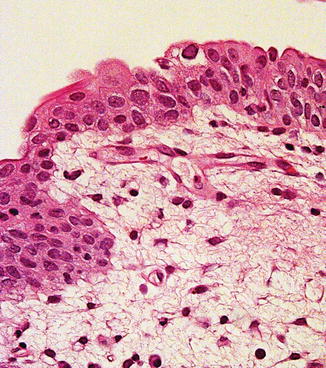
Fig. 2.24
Polyomavirus infection in urothelium following renal transplant
Pathologists must be aware of the diagnostic pitfalls and exercise caution when evaluating urothelial atypia following treatment with chemotherapy or irradiation. In most cases, knowledge of the prior treatment is crucial to correctly diagnosing the epithelial and stromal changes present.
If the distinction between treatment-induced atypia and dysplasia/carcinoma-in-situ is uncertain, a conservative approach with repeat cystoscopy and biopsy is indicated, preferably after the inflammation has subsided.
Cyclophosphamide therapy may induce stromal fibrosis, vascular intimal thickening, mural fibrin deposition in vessels, and vascular ectasia. It also induces epithelial necrosis followed by rapid atypical regeneration. The metabolic effects of cyclophosphamide including arrest of cell and nuclear division produce bi- and multi-nucleated cells, often with large bizarre nuclei resembling radiation injury changes that can be mistaken for malignancy.
Cyclophosphamide may also induce reactivation of polyomavirus infection causing marked nuclear atypia in the surface urothelium. In rare cases, polyomavirus infection (BK virus) infection mimics flat intraepithelial lesions.
In BCG treated bladders, residual carcinoma in situ might only be present in von Brunn nests. Loss of intercellular cohesion in carcinoma in situ may result in the so-called “denuding cystitis” or in residual neoplastic cells loosely attached to the surface (“clinging” pattern). Also of importance are the reactive changes associated with BCG therapy, which include both acute and chronic inflammation. There may also be a pattern of reactive epithelial atypia and granulomatous reaction in the bladder wall.
Mitomycin C and thiotepa, when used as topical chemotherapeutic agents in the bladder, produce identical histologic changes. These include exfoliation, epithelial denudation, multinucleation, cytoplasmic vacuolization, and the appearance of bizarre, non-malignant nuclei in the superficial layer of the urothelium.
A marked necro-inflammatory process follows administration of topical mitomycin C. There is a histiocytic response extending deep into the bladder wall. Mitomicin C may also initiate eosinophilic cystitis, a useful clue for the surgical pathologist when evaluating small bladder biopsies in this setting. These agents are not metabolic inhibitors of DNA replication and thus do not produce full-thickness urothelial atypia, as is seen after cyclophosphamide therapy.
Patients receiving ketamine may present reactive urothelial changes that can mimic urothelial CIS.
Radiation therapy produces a variety of bladder lesions associated with a progression of pathologic findings. The earliest change, usually seen after 3–6 weeks, consists of acute cystitis with desquamation of urothelial cells and hyperemia with edema of the lamina propria. The urothelium shows varying degrees of atypia, including cytoplasmic and nuclear vacuolization, karyorrhexis, stromal hyalinization, thrombosis of blood vessels, and mesenchymal cell atypia similar to that seen in giant cell cystitis.
Enlarged nuclei may have large nucleoli, and degenerative nuclear features are usually present. Surface ulceration with fibrin deposition, or a reactive, tumor-like epithelial proliferation associated with fibrosis of the lamina propria and/or muscularis propria, arteriolar mural thickening and hyalinization, and atypical and sometimes multinucleated stromal cells are features seen in late cases of radiation cystitis, usually becoming evident months or years after radiation therapy.
An important long term effect of radiotherapy is the development of de novo radiation induced bladder cancer which usually is a urothelial carcinoma; occasionally it is a squamous cell neoplasm. Rare examples of sarcomatoid carcinoma (or carcinosarcoma) and sarcoma of the urinary bladder have been reported.
In addition, the damaged mucosa may become ulcerated, with adjacent atypical regenerating urothelium showing pseudoepitheliomatous hyperplasia. This change is more common after ulceration related to radiation therapy.
2.2.3 Urothelial Carcinoma
General features
Bladder cancer is the 7th most common cancer worldwide, accounting for approximately 336,000 new cases each year. There are significant variations in incidence, morbidity, and mortality rates of bladder cancer in different countries and ethnicity groups.
African American men have a much lower incidence of bladder cancer, but their mortality rates are similar to white Caucasians. Bladder cancer occurs two to five times more frequently in men than women. This has been attributed to different smoking and occupational exposure between men and women.
The majority of bladder cancer patients present with hematuria. Approximately 20 % of patients being evaluated for gross hematuria will subsequently be diagnosed with bladder cancer. Similarly, of patients presenting with microscopic hematuria, up to 10 % will be diagnosed with bladder cancer.
A significant proportion of patients also have irritative voiding symptoms, including urgency, frequency and dysuria, their symptoms are mistakenly attributed to urinary tract infection.
Field cancerization and tumor multicentricity
Development of multifocal tumors in the same patient, either synchronous or metachronous, is a common characteristic of urothelial malignancy. Multiple coexisting tumors have often arisen before clinical symptoms are apparent. The separate tumors may or may not share a similar histology. Two theories have been proposed to explain the frequency of urothelial tumor multifocality.
One theory, the monoclonal theory, suggests that the multiple tumors arise from a single transformed cell which proliferates and spreads throughout the urothelium either by intraluminal implantation or by intraepithelial migration.
The second theory, the field-effect theory, explains tumor multifocality as a development secondary to field cancerization effect.
Histologic grading (1998 International Society of Urological Pathology (ISUP)/2004 World Health Organization (WHO) Classification)
In 1998, a revised system of classifying noninvasive papillary urothelial neoplasms of the urinary bladder was proposed. This system was subsequently formally adopted by the World Health Organization (2004).
This new system separates noninvasive papillary urothelial neoplasms into 4 categories, designated papilloma, papillary urothelial neoplasm of low malignant potential (PUNLMP), low grade carcinoma, and high grade carcinoma.
Papillary urothelial neoplasm of low malignant potential (PUNLMP)
A PUNLMP is a low-grade urothelial tumor with a papillary architecture and a purported low incidence of recurrence and progression. This lesion is histologically defined by the WHO (2004) classification system as a papillary urothelial tumor which resembles the exophytic urothelial papilloma, but with increased cellular proliferation exceeding the thickness of normal urothelium. All such tumors would have been considered grade 1 urothelial carcinomas by the WHO 1973 grading system (Table 2.5).
Table 2.5
Proposals to histological grading of urothelial carcinoma of the urinary bladder
1973 WHO
1998 WHO/ISUP
1999 WHO
2004 WHO
Current Proposal
Papilloma
Papilloma
Papilloma
Papilloma
Papilloma
Grade 1
PUNLMP
PUNLMP
PUNLMP
Grade 1 (low grade)
Grade 2
Low grade
Grade 1
Low grade
Grade 2 (low grade)
Grade 2
Grade 3 (high grade)
Grade 3
High grade
Grade 3
High grade
Grade 4 (high grade)
Cytologic atypia is minimal or absent and architectural abnormalities are minimal with preserved polarity. Mitotic figures are infrequent and usually limited to the basal layer. Clinically, these tumors show a male predominance (3:1) and occur at a mean age of 65 years. They are most commonly identified during investigation of gross or microscopic hematuria. Cystoscopically, these lesions are typically 1–2 cm in greatest dimension, and located on the lateral wall of the bladder or near the ureteric orifices (Fig. 2.25)
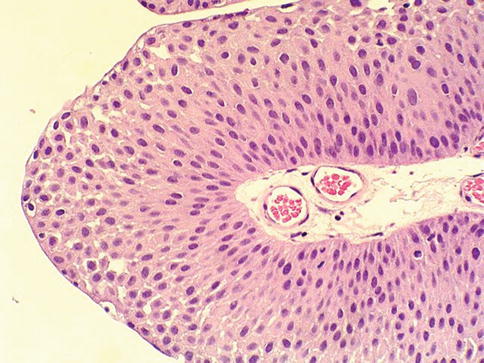
Fig. 2.25
Microscopic appearance of papillary urothelial neoplasia of low malignant potential
The published recurrence and progression rates show about 30 % recurrence and about 3 % progression rate. Reproducibility between observers in diagnosis of PUNLMP is low, and therefore, it remains a controversial category.
Low grade urothelial carcinoma
A low grade papillary urothelial carcinoma shows fronds with recognizable variation in architecture and cytology. The tumor shows slender papillae with frequent branching and variation in nuclear polarity; nuclei show enlargement and irregularity; chromatin is vesicular and nucleoli are often present (Fig. 2.26)
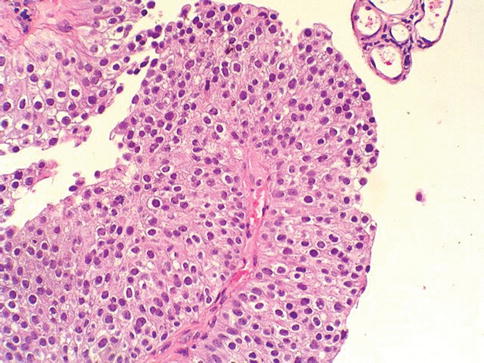
Fig. 2.26
Microscopic appearance of low grade urothelial carcinoma
Mitotic figures may occur at any level in low grade papillary urothelial carcinoma. Such cases would have been considered as grade 1 or grade 2 in the WHO (1973) classification scheme. Altered expression of cytokeratin 20, CD44, p53 and p63 is frequent. Some tumors are diploid, but aneuploidy is the rule.
FGFR3 mutations are seen with about the same frequency as in PUNLMP. The male: female ratio is 2.9:1 and the mean age is 70 years (range 28–90 years). Most patients present with hematuria and have a single tumor in the posterior or lateral bladder wall.
However, 22 % of patients with low grade papillary urothelial carcinoma have two or more tumors. Tumor recurrence, stage progression and tumor-related mortality are 50, 10 and 5 %, respectively.
High grade urothelial carcinoma
In high grade papillary urothelial carcinoma, the cells lining papillary fronds show obviously disordered arrangement with cytologic atypia (Fig. 2.27) All tumors classified as grade 3 in the 1973 WHO scheme, as well as some tumors assigned grade 2 in that classification, would be considered high grade carcinoma in the 2004 WHO classification.
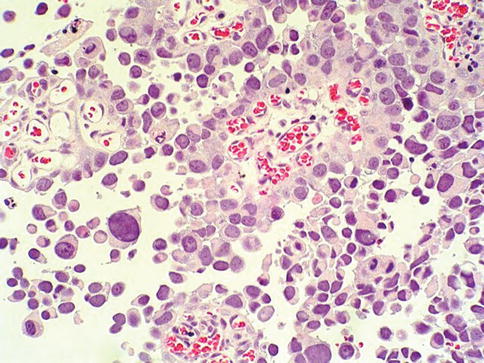
Fig. 2.27
Microscopic appearance of high grade urothelial carcinoma
The papillae are frequently fused. Both architectural and cytologic abnormality are recognizable at scanning power. The nuclei are pleomorphic with prominent nucleoli and altered polarity. Mitotic figures are frequent. The thickness of the urothelium varies considerably.
Carcinoma-in-situ is frequently evident in the adjacent mucosa. Changes in cytokeratin 20, p53 and p63 expression, as well as aneuploidy are more frequent than in low grade lesions. Molecular alterations in these tumors include over-expression of p53, HER2 or EGFR, and loss of p21Waf1 or p27kip1 as seen with invasive cancers.
Genetically, high-grade non-invasive lesions (pTa G3) resemble invasive tumors. A comparative genomic hybridization-based study showed deletions at 2q, 5q, 10q, and 18q as well as gains at 5p and 20q.
Hematuria is common and the endoscopic appearance varies from papillary to nodular or solid. There may be single or multiple tumors. Stage progression and death due to disease are observed in as many as 65 % of patients.
Histologic grading (1973 World Health Organization (WHO) Classification)
Histologic grading is one of the most important prognostic factors in bladder cancer. The first widely accepted grading system for papillary urothelial neoplasms, that is still in use in many institutions, was the WHO (1973) classification system.
It has four categories: papilloma, grade 1 carcinoma, grade 2 carcinoma, and grade 3 carcinoma. Histologic grading is based on the degree of cellular anaplasia with grade 1 tumors having the least degree of anaplasia compatible with a diagnosis of malignancy, grade 3 tumors have the most severe degree of anaplasia, and grade 2 tumors have an intermediate degree of cellular anaplasia.
Anaplasia is further defined by the authors of the WHO (1973) classification as increased cellularity, nuclear crowding, disturbed cellular polarity, failure of differentiation from the base to the surface, nuclear polymorphism, irregularity cell size, variations in nuclear shape and chromatin pattern, displaced or abnormal mitotic figures, and giant cells. Urothelial papilloma will be discussed later under benign tumors.
Grade 1 urothelial carcinoma
Grade 1 papillary carcinoma consists of an orderly arrangement of normal urothelial cells lining delicate papillae with minimal architectural abnormality and minimal nuclear atypia. Nuclear grooves are usually present. There may be some complexity and fusion of the papillae, but this is usually not prominent. The urothelium is often thickened to more than 7 cell layers.
The urothelium displays normal maturation and cohesiveness, with an intact superficial cell layer. The nuclei tend to be uniform in shape and spacing, although there may be some enlargement and elongation. The chromatin texture is finely granular, without significant nucleolar enlargement. Mitotic figures are rare or absent, and basally located. Grade 1 tumor should be distinguished from urothelial papilloma which is a benign lesion.
Grade 2 urothelial carcinoma
Grade 2 carcinoma represents a broad group of tumors encompassing a spectrum of cytologic atypia and some variability in the relative proportions of cells with atypical features. Grade 2 carcinomas retain some of the orderly architectural appearance and maturation of grade 1 carcinoma, but display at least focal moderate variation in orderliness, nuclear appearance, and chromatin texture apparent at low magnification.
Cytologic abnormalities are invariably present in grade 2 carcinoma, with moderate nuclear crowding, moderate loss of cell polarity, moderate nuclear hyperchromasia, moderate anisonucleosis, and mild nucleolar enlargement. Mitotic figures are usually limited to the lower half of the urothelium, but this is an inconstant feature. Superficial cells are usually present, and the urothelial cells are predominantly cohesive, although variation in cohesion may be present.
Some tumors may be extremely orderly, reminiscent of grade 1 carcinoma, with only a small focus of obvious disorder or irregularity. These are considered grade 2 cancer, recognizing that tumor grade is based on the highest level of abnormality present.
The prognosis for patients with grade 2 urothelial carcinoma is significantly worse than for those with lower grade papillary cancer. Recurrence risk for patients with non-invasive grade 2 cancer is 45–67 %. Invasion occurs in up to 20 %.
Grade 3 urothelial carcinoma
Grade 3 carcinoma displays the most extreme nuclear abnormality of any papillary urothelial cancer, similar to changes observed in urothelial carcinoma in situ. The obvious urothelial disorder and loss of polarity is present at scanning magnification. The superficial cell layer is partially or completely absent with grade 3 carcinoma, accompanied by prominent cellular dyscohesion.
There is obvious loss of normal architecture and cell polarity, and frequent atypical mitotic figures. Cellular anaplasia, characteristic of grade 3 carcinoma, is defined as increased cellularity, nuclear crowding, random cellular polarity, absence of normal mucosal differentiation, nuclear pleomorphism, irregularity in cell size, variation in nuclear shape, capricious chromatin pattern, increased frequency of mitotic figures and occasional neoplastic giant cells.
Recurrence risk for patients with non-invasive grade 3 cancer is 65–85 %, with invasion occurring in 20–52 % and cancer-specific death in up to 35 % following surgical treatment. Of surgically treated patients with grade 3 cancer and lamina propria invasion, 46–71 % develop recurrences, 24–48 % develop muscle-invasive cancer, and 25–71 % suffer cancer-specific death, emphasizing a need for appropriated treatment of these patients.
Histologic Grading of Urothelial Carcinoma: The Four-tier Proposal
Recently, a four-tier proposal has been reported. In this proposal, noninvasive papillary urothelial carcinomas are separated into four categories: grade 1 urothelial carcinoma (low grade), grade 2 urothelial carcinoma (low grade), grade 3 urothelial carcinoma (high grade), and grade 4 urothelial carcinoma (high grade) (Table 2.6). PUNLMP is classified as “grade 1 urothelial carcinoma (low grade)” in the four-tier grading scheme. Urothelial papilloma remains as a benign urothelial tumor following identical diagnostic criteria and terminology to those defined in the 1973 and 2004 WHO classification.
Table 2.6
Histological criteria for the proposed grading system of urothelial carcinoma of the bladder (4-tier system)
Characteristics
Grade 1(low grade)
Grade 2(low grade)
Grade 3(high grade)
Grade 4(high grade)
Increased cell layers (>7)
Yes
Variable
Variable
Variable,usually <7 layers
Superficial umbrella cells
Present
Often present
Usually absent
Usually absent
Polarity/overall architecture
Normal
Mildly distorted
Moderately distorted
Severely distorted
Discohesiveness
Normal
Normal
Mild to moderate
Severe
Clear cytoplasm
May be present
May be present
Usually absent
Usually absent
Nuclear size
Normal or slightlyincreased
Mildly increased
Moderately increased
Markedly increased
Nuclear pleomorphism
Uniform, slightlyelongated to oval
Mild, round to ovalwith slight variationin shape and contour
Moderate
Marked
Nuclear polarization
Normal to slightly abnormal
Abnormal
Abnormal
Absent
Nuclear hyperchromasia
Slight or minimal
Mild
Moderate
Severe
Nuclear grooves
Present
Present
Absent
Absent
Nucleoli
Absent orinconspicuous
Inconspicuous
Enlarged,often prominent
Multiple prominent nucleoli
Mitotic figures
None/rare,basal location
May be present,at any level
Often present
Prominent and frequent,atypical forms
Stromal invasion
Rare
Uncommon
May be present
Often present
Grade 1 Urothelial Carcinoma (Low Grade)
The diagnostic criteria are identical to those defined in the 1998 WHO/ISUP and 2004 WHO classification for PUNLMP. We propose to change the terminology of PUNLMP to “grade 1 urothelial carcinoma (low grade).” In these tumors, cytologic atypia is minimal or absent and architectural abnormalities are slight with preserved polarity. Mitotic figures are infrequent and usually limited to the basal layer. Grade 1 tumor should be distinguished from urothelial papilloma, which is a benign lesion without invasive potential or risk of progression. The key difference between papilloma and grade 1 urothelial carcinoma (low grade) is the number of epithelial layers covering the papillae.
Grade 2 Urothelial Carcinoma (Low Grade)
The diagnostic criteria are identical to those defined in the 1998 WHO/ISUP and 2004 WHO classification for low grade urothelial carcinomas. These tumors are characterized by an overall orderly appearance but with areas of variation in architectural and cytologic features recognizable at scanning power. They are differentiated from grade 1 urothelial carcinoma (low grade) by the presence of easily recognizable cytologic atypia including variation of polarity and nuclear size, shape, and chromatin texture. Mitotic figures are infrequent and may be seen at any level of the urothelium.
Grade 3 Urothelial Carcinoma (High Grade)
We feel that the spectrum of high grade urothelial carcinomas under the 2004 WHO classification scheme is quite broad and there is a need to separate these tumors for further investigation. Grade 3 urothelial carcinomas (high grade) display an intermediate degree of architectural and cytologic abnormality between grade 2 urothelial carcinomas (low grade) and grade 4 urothelial carcinomas (high grade). Architectural disorder in these tumors is obvious, with branching and bridging of papillary projections. Nevertheless, a certain degree of polarity and nuclear uniformity are still discernible. Severe anaplasia is not seen in these tumors. All grade 3 urothelial carcinomas would be classified as high grade urothelial carcinoma using the 2004 WHO classification scheme.
Grade 4 Urothelial Carcinoma (High Grade)
Cases with severe nuclear anaplasia are considered grade 4 urothelial carcinoma in the current proposal. These tumors present an overall impression of complete architectural disorder with absence of polarity, loss of superficial umbrella cells, and marked variation of all nuclear parameters. Numerous irregularly distributed mitotic figures are frequently noted. Severe cytologic atypia is usually uniformly present in all fields or all histologic sections examined. Unlike grade 1 or grade 2 urothelial carcinoma (low grade), these tumors often have less than seven layers in thickness. There is remarkable cellular discohesiveness. These cases are typically associated with stromal invasion and advanced stage bladder cancer.
Aggressive variants of urothelial carcinoma, including nested variant, micropapillary variant, plasmacytoid variant, sarcomatoid carcinoma, small cell carcinoma, large cell undifferentiated carcinoma, pleomorphic giant cell carcinoma, should also be graded as grade 4 tumor in the current grading scheme.
Tumor heterogeneity
The WHO (2004)/ISUP system provides clearly defined histologic criteria for each of its diagnostic categories; however, urothelial neoplasms frequently demonstrate features of more than one grade (grade heterogeneity). The grading of papillary urothelial tumors is typically based on the worst grade present.
Invasive urothelial carcinoma
General features
At gross examination, most tumors present as a single, solid, polypoid mass with or without ulceration, and may also appear sessile and extensively infiltrate the bladder wall.
Histologically, the neoplastic cells invade the bladder wall as nests, cords, trabeculae, small clusters, or single cells that are often separated by a desmoplastic stroma. The tumor sometimes grows in a more diffuse, sheet-like pattern, but even in these cases, focal nests and clusters are generally present.
The cells show moderate to abundant amphophilic or eosinophilic cytoplasm and large hyperchromatic nuclei. In larger nests, palisading of nuclei may be seen at the edges of the nests. The nuclei are typically pleomorphic and have irregular contours with angular profiles. Nuclear grooves may be identified in some cells. Nucleoli are highly variable in number and appearance (Fig. 2.28)
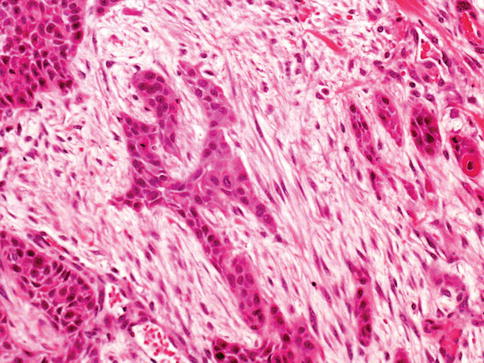
Fig. 2.28
High grade urothelial carcinoma infiltrating múscularis propria and desmoplastic stroma
Some cells contain single or multiple small nucleoli and others have large eosinophilic nucleoli. Foci of marked pleomorphism may be seen, with bizarre and multinuclear tumor cells present. Mitotic figures are common, including many abnormal forms. Invasive tumors are most commonly high grade, usually showing marked anaplasia with focal giant cell formation.
Pathologic stage is most critical for assessing patient prognosis. The 2010 TNM (tumor, lymph node, and metastasis) staging information should be provided in the pathology report.
Nonmuscleinvasive bladder carcinoma (T1 tumor)
Infiltrating urothelial carcinoma is defined by the World Health Organization (WHO) as a urothelial tumor that invades beyond the basement membrane. The 2010 TNM staging system defines T1 tumors of the bladder as those invading the lamina propria/submucosa, but not the muscularis propria.
The recognition of lamina propria invasion by urothelial carcinoma is one of the most challenging fields in surgical pathology, and the pathologist should follow strict criteria in its assessment (Tables 2.7 and 2.8), as follows:
Table 2.7
Histologic features that are useful for the diagnosis of stromal invasion
Histologic grade:
Invasive cells are usually higher nuclear grade
Invading epithelium
Irregularly shaped nests
Single cell infiltration
Irregular or absent basement membrane
Tentacular finger-like projections
Paradoxical differentiation
Angiolymphatic invasion
Stromal response
Desmoplasia or fibrotic stroma
Retraction artifact
Inflammation
Myxoid stroma
Pseudosarcomatous stroma
Table 2.8
Pitfalls in the diagnosis of stage pT1 urothelial carcinoma
Tangential sectioning and poor orientation
Obscuring inflammation
Thermal injury
Urothelial carcinoma in situ involving von Brunn’s nests
Muscle invasion indeterminate for type of muscle (muscularis propria versus muscularis mucosae)
Variants of urothelial carcinoma with deceptively bland cytology
Pseudoinvasive nests of benign proliferative urothelial lesions
Histologic grade:
While invasion may be present in low-grade tumors, it is much more commonly encountered in high grade lesions, reaching 70–96 % in some series (Figs. 2.29, 2.30, 2.31, 2.32, and 2.33)
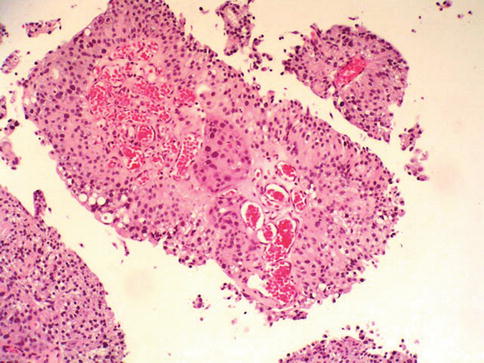
Fig. 2.29
High grade urothelial carcinoma with invasion into stalk and paradoxical differentiation of the invasive nest
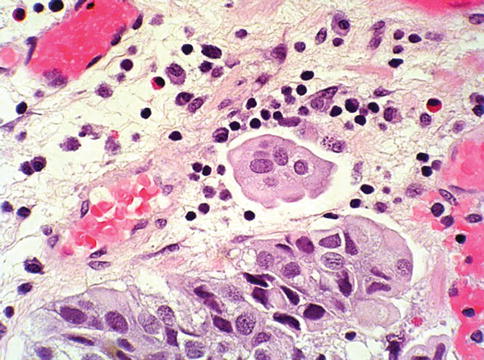
Fig. 2.30
Infiltrating tumor nests with retraction artefact
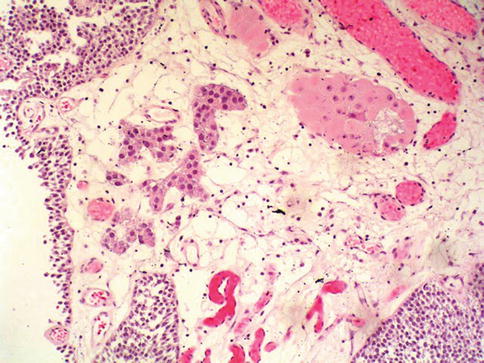
Fig. 2.31
Low grade urothelial carcinoma with early invasion into stalk
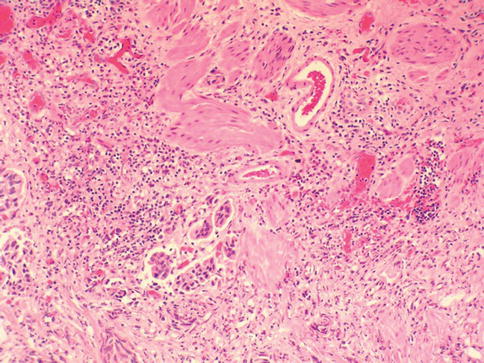
Fig. 2.32
Relationship of invading nests with múscularis mucosae
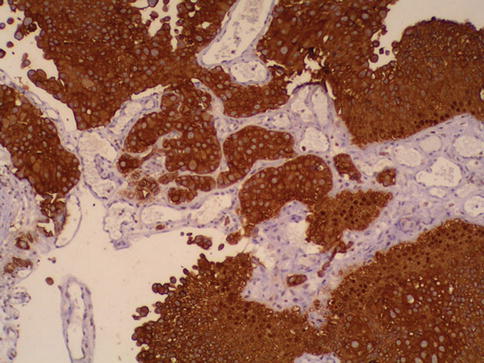
Fig. 2.33
Keratin staining highlights invading carcinoma nests
Stroma-epithelial interface: Tangentially sectioned, densely packed non-invasive papillary tumors exhibit a stroma-epithelial interface that is smooth and regular. In instances of true invasion, one is likely to see variable sized and irregularly shaped nests or individual tumor cells insinuating through the stroma.
When the specimen includes tangential sections through non-invasive tumor or when urothelial carcinoma involves von Brunn’s nests, the basement membrane preserves a regular contour, whereas it is frequently absent or disrupted in cases of true invasion.
The smoothness of the epithelial-stromal interface may be assessed on H&E stains. In some cases, however, additional findings may be helpful; for example, there is a parallel array of thin-walled vessels that evenly line the basement membrane of non-invasive nests. These are absent in patients with invasive tumors.
Invading epithelium:
The invasive front of the neoplasm may show one of several features. Most commonly, tumors invade the underlying stroma as single cells or irregularly shaped nests of tumor cells. Sometimes tentacular or finger-like extensions can be seen arising from the base of the papillary tumor.
Frequently, the invading nests appear cytologically different from cells at the base of the non-invasive component. Invasive tumor cells often have more abundant cytoplasm and a higher degree of nuclear pleomorphism. In some cases, particularly in microinvasive disease, the invasive tumor cells may acquire abundant eosinophilic cytoplasm.
At low to medium power magnification, these microinvasive cells seem to be more differentiated than the overlying non-invasive disease, a feature known as paradoxical differentiation.
Stromal response:
The stromal response to invading carcinoma is not always uniformly present in invasive urothelial carcinoma, and the diagnosis of invasion may rely on identification of the typical characteristics of the invading epithelium. The stromal reaction in the lamina propria associated with invasive tumor may be inflammatory, myxoid, or fibrous.
Assessment of differences in stromal growth pattern provides an important diagnostic clue. Although the majority of bladder tumors with unquestionable lamina propria invasion exhibit some sort of stromal reaction, microinvasive disease usually does not, making its identification even more difficult. In some cases, retraction artifact around superficially invasive individual tumor cells may mimic angiolymphatic invasion.
Often, this finding is focal, and may itself be one of the early signs of invasion into the lamina propria. Lamina propria invasion may elicit a brisk inflammatory response. Numerous inflammatory cells in the lamina propria often obscure the interface between epithelium and stroma. This makes small nest or single cell invasion difficult to recognize. Cytokeratin immunostaining is useful in difficult cases.
Invasive urothelial carcinoma may have a cellular stroma with spindled fibroblasts and variable collagenization, or a hypocellular stroma with myxoid background.
Rarely, the tumor induces an exuberant proliferation of fibroblasts, which may display alarming cellular atypia similar to giant cell cystitis. This feature, although a helpful clue to invasion, should not be mistaken for the spindle cell component of sarcomatoid urothelial carcinoma.
Immunostains for cytokeratin are helpful in difficult cases though some myofibroblasts may also be positive for keratin. The proliferating stroma is usually nonexpansile, being limited to areas around the neoplasm, and is composed of cells which have a degenerate or smudged appearance.
Diagnostic pitfalls:
Transuretheral resection specimens are excised in a piecemeal fashion. Submitted tissue fragments are of variable shape and size and are difficult if not impossible to orient properly (Table 2.8). Furthermore, due to their complex architecture, papillary tumors are inevitably tangentially sectioned in multiple planes, resulting in the presence of isolated nests of non-invasive tumor cells within connective tissue. Smooth, round, and regular contours favor tangential sectioning, whereas irregular, jagged nests with haphazard arrangement favor stromal invasion.
Diagnosis of invasion in some of these cases can be facilitated by immunohistochemical study with anti-cytokeratin antibodies. Thermal injury or cautery artefact produces severely distorted morphology, rendering accurate diagnosis of invasion difficult.
Tumor cells involving von Brunn’s nests may also mimic lamina propria invasion. This is especially problematic when von Brunn’s nests are prominent or when they have been distorted by inflammatory or cautery artifact.
Substaging of T1 tumors:
The recurrence and progression rates for T1 tumors are highly variable. There is need for an accurate, easy-to-use, reproducible substaging system to stratify pT1 patients into different prognostic groups.
Muscularis mucosae consists of thin and wavy fascicles of smooth muscle frequently associated with large, thin walled blood vessels. Muscularis mucosae can be identified in 15–83 % of biopsy specimens,; however, 6 % of the radical resection specimens do not have discernable muscularis mucosae. Thus, the “large” vessels have been used as a surrogate marker of muscularis mucosae in all published studies that have proposed T1 substaging based on muscularis mucosae invasion. Some authors have raised concerns about the practicality and validity of substaging pT1 disease based on assessment of muscularis mucosa invasion, and currently this practice is not universally
Stage pT2 tumor
The 2010 TNM staging system subclassifies pT2 tumor into two categories: cancer invading less than one-half of the depth of muscular propria (pT2a) and cancer invading greater than one-half of the depth of muscularis propria (Table 2.9). Tumors with an infiltrative growth pattern are associated with a worse prognosis than tumor displaying a non-infiltrative (nodular or trabecular) growth pattern.
Table 2.9
TNM classification of bladder cancer (2010 revision)
Primary tumor
TX
Primary tumor can not be assessed
T0
No evidence of primary tumor
Ta
Non-invasive papillary carcinoma
Tis
Carcinoma in situ: “flat tumor”
T1
Tumor invades subepithelial connective tissue (lamina propria)
T2
Tumor invades muscularis propria
T2a
Tumor invades superficial muscle (inner half)
T2b
Tumor invades deep muscle (outer half)
T3
Tumor invades perivesical tissue
T3a
Microscopically
T3b
Macroscopically (extra-vesical mass)
T4
Tumor invades any of the following: prostate, uterus, vagina, pelvic wall, and abdominal wall
T4a
Tumor invades prostate or uterus or vagina
4b
Tumor invades pelvic wall or abdominal wall
The suffix “m” should be added to the appropriate T category to indicate multiple tumors. The suffix “is” may be added to any T to indicate the presence of associated carcinoma in situ
Regional Lymph Nodes (N)
NX
Regional lymph nodes cannot be assessed
N0
No regional lymph node metastasis
N1
Single regional lymph node metastasis in the true pelvis (hypogastric, obturator, external iliac, or presacral lymph node)
N2
Multiple regional lymph node metastasis in the true pelvis (hypogastric, obturator, external iliac, or presacral lymph node )
N3
Lymph node metastasis to the common iliac lymph nodes
Distant Metastasis (M)
M0
No distant metastasis
M1
Distant metastasis
Stage pT3 tumor
Stage pT3 bladder carcinomas is defined as tumor invading into perivesical soft tissue. The subdivision of pT3 tumors into T3a (tumors with microscopic extravesical tumor extension) and pT3b (tumors with gross extravesical extension).
Stage pT4 tumor
Stage pT4 bladder cancer is defined as tumor invading into adjacent organ including the uterus, vagina, prostate. pelvic wall or abdominal wall.
Cancers with prostatic involvement that penetrated the full thickness of bladder wall to involve the prostate vs. involved the prostate by extension from the prostatic urethra. Five-year overall survivals were 21 and 55 % for the former or later group of patients, respectively.
Histologic variants of invasive bladder cancer
Urothelial carcinoma has a propensity for divergent differentiation. Virtually the whole spectrum of bladder cancer variants described below may be seen in variable proportions in otherwise typical urothelial carcinoma or in its pure forms (Table 2.10).
Table 2.10
Histologic variants of urothelial carcinoma
Urothelial carcinoma with mixed differentiation (specify type and percentage)
With squamous cell differentiation
With glandular differentiation
Other
Urothelial carcinoma, nested variant
With small tubules
Urothelial carcinoma, large nests variant
Urothelial carcinoma, inverted growth variant
Urothelial carcinoma, micropapillary variant
Urothelial carcinoma, microcystic variant
Lymphoepithelioma-like (urothelial) carcinoma
Plasmacytoid urothelial carcinoma
Urothelial carcinoma, clear cell (glycogen-rich) variant
Urothelial carcinoma, lipoid cell variant
Urothelial carcinoma with syncytiotrophoblastic giant cells
Sarcomatoid urothelial carcinoma
Small cell carcinoma
Large cell neuroendocrine carcinoma
Urothelial carcinoma with myxoid stroma
Urothelial carcinoma with rhabdoid features
Urothelial carcinoma in augmentation cystoplastia
Large cell undifferentiated carcinoma
Giant cell carcinoma
With osteclast-type giant cells
NOS (not otherwise specified)
Urothelial carcinoma with unusual stromal reactions
Pseudosarcomatous stroma
Stromal osseous or cartilaginous metaplasia
Osteoclast-type giant cells
Prominent lymphoid infiltrate
Urothelial carcinoma with squamous differentiation
About 20 % of urothelial carcinomas contain areas of squamous or glandular differentiation. Squamous differentiation, defined by the presence of intercellular bridges or keratinization, occurs in 21 % of urothelial carcinomas of the bladder. Its frequency increases with grade and stage. Some cases have urothelial carcinoma in situ as the only urothelial component (Figs. 2.34 and 2.35).
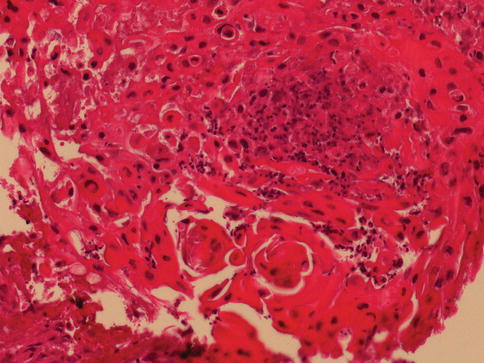
Fig. 2.34
Urothelial carcinoma with prominent keratinizing squamous cell differentiation
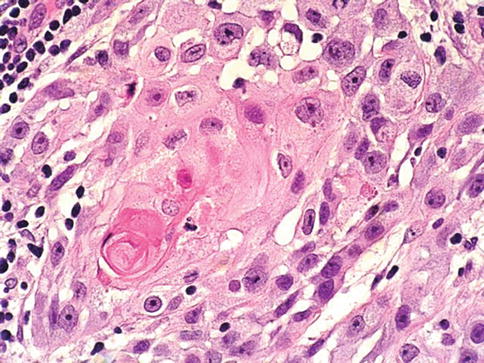
Fig. 2.35
Urothelial carcinoma with focal squamous cell differentiation
Cases with areas of squamous differentiation may have a less favorable response to therapy than pure urothelial carcinoma.
Low-grade urothelial carcinoma with focal squamous differentiation has a higher recurrence rate than pure low grade urothelial carcinoma. Tumors with any identifiable urothelial element are classified as urothelial carcinoma with squamous differentiation, and an estimate of the percentage of squamous component should be provided.
Urothelial carcinoma with squamous differentiation may express urothelial (S100P 83 %, GATA3 35 %, uroplakin III 13 %) and squamous associated markers CK14 87 % and desmoglein-3 70 %)
Urothelial carcinoma with glandular differentiation
Glandular differentiation is less common than squamous differentiation and may be present in about 6 % of urothelial carcinomas of the bladder. Glandular differentiation is defined by the presence of true glandular spaces within the tumor. These may be tubular or enteric glands with mucin secretion.
A colloid-mucinous pattern characterized by nests of cells “floating” in extracellular mucin, occasionally with signet ring cells, may be present. Cytoplasmic mucin-containing cells are present in 14–63 % of typical urothelial carcinoma and are not considered to represent glandular differentiation. The diagnosis of adenocarcinoma is reserved for pure tumors.
A tumor with mixed glandular and urothelial differentiation is classified as urothelial carcinoma with glandular differentiation, and an estimate of the percentage of glandular component should be provided. A form of glandular differentiation with villous–like appearance has been described associated with papillary urothelial carcinoma (Figs. 2.36 and 2.37).
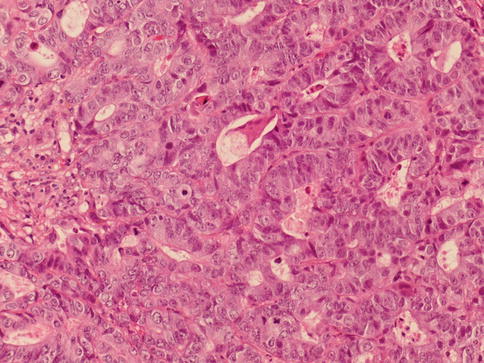
Fig. 2.36
Urothelial carcinoma with glandular differentiation
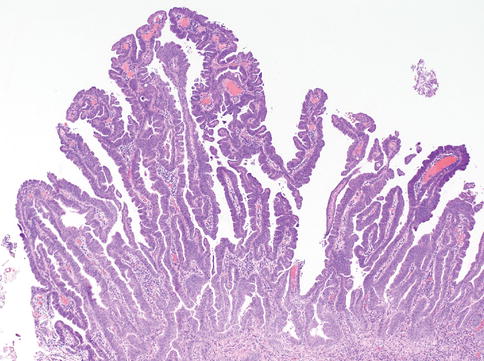
Fig. 2.37
Urothelial carcinoma with villous-like glandular differentiation
The expression of MUC5AC-apomucin may be useful as immunohistochemical marker of glandular differentiation in urothelial tumors.
When adenocarcinoma is present in association with urothelial carcinoma, even focally, it portends a poor prognosis.
Urothelial carcinoma, nested variant
The nested variant of urothelial carcinoma is an aggressive neoplasm. There is a marked male predominance, and 70 % of patients die 4–40 months after diagnosis, in spite of therapy. This rare pattern of urothelial carcinoma was first described as a tumor with a “deceptively benign” appearance that closely resembles von Brunn’s nests infiltrating the lamina propria. A variable proportion of infiltrating cell nests may exhibit small tubular lumens (Tables 2.11, 2.12, and 2.13).
Table 2.11
Diverse morphologic manifestations and pitfalls in the diagnosis of urothelial carcinoma variants
Feature of urothelial carcinoma
Benign lesion in differential diagnosis
Nests (including occasional small lumens)
Von Brunn’s nests, cystitis glandularis
Small tubules
Nephogeneic metaplasia, von Brunn’s nests, cystitis glandularis
Glands of mediumsize
Cystitis glandularis, cystitis cystica
Cysts (microcysts)
Cystitis cystica, cystitis cystica et glandularis
Inverted growth
Inverted papilloma
Table 2.12
Variants of urothelial carcinoma according to their differential diagnosis
Variants of urothelial carcinoma
Main differential diagnosis
Urothelial carcinoma with squamous and/or glandular differentiation
Squamous cell carcinoma, adenocarcinoma
Urothelial carcinoma, nested variant (including small tubules type)
Von Brunn’s hyperplasia
Urothelial carcinoma, inverted variant
Inverted papilloma
Urothelial carcinoma, micropapillary variant
Adenocarcinoma, serous carcinoma of the ovary
Urothelial carcinoma, microcystic variant
Cystitis cystica and cystistis glandularis, rarely adenocarcinoma
Lymphoepithelioma-like (urothelial) carcinoma
Lymphoma
Urothelial carcinoma, plasmacytoid variant
Plasmacytoma
Urothelial carcinoma, clear cell (glycogen-rich) variant
Clear cell carcinomas from kidney and other sites
Urothelial carcinoma, lipoid cell variant
Carcinosarcoma/sarcomatoid carcinoma of hetorologous type (liposarcoma)
Urothelial carcinoma with syncitiotrophoblastic giant cells
Choriocarcinoma either primary or secondary
Large cell undifferentiated carcinoma
Metastasis from other sites
Urothelial carcinoma with rhabdoid feature
Plasmacytoma, inflammatory myofibroblastic tumor, metastasis from other sites
Urothelial carcinoma with peudosarcomatous stroma
Sarcomatoid carcinoma
Urothelial carcinoma with stromal osseous or cartilaginous metaplasia
Sarcomatoid carcinoma with hetorologous element
Urothelial carcinoma with osteoclast-type giant cells
Reactive granulomatous lesion
Urothelial carcinoma with prominent lymphoid infiltrate
Lymphoma, lymphoepithelial carcinoma
Table 2.13
Key features in the differential diagnosis of the nested variant of urothelial carcinoma
Lumenformation
Marked cytologic atypia in deeperportion
Infiltrativebase
Muscleinvasion
Immunohistochemistry
Nested variant
Present, variable
Present, frequent
Present, frequent
Present, frequent
Low p27kip1, high MIB1proliferation index
Florid von Brunn nests
Present, variable
Absent
Absent
Absent
Variable
Nephrogenicmetaplasia
Present
Absent
Present, frequent
Present,rare
Variable
Cystitis cystica,cystitis glandularis
Present
Absent
Absent
Absent
Variable
Paraganglionic tissueand paraganglioma
Absent, associatedprominent vascularnetwork
Absent
Absent
Present
Neuroendocrinemarkers positive
A recent study based on 56 cases of the nested variant showed that architectural pattern of the tumor varied from solid expansile to infiltrative nests characterized by deceptively bland histologic features resembling von Brunn nests. Typical features of high grade conventional urothelial carcinoma were present in 32 cases. Most neoplastic cells had nuclei of low to intermediate nuclear grade with occasional nuclear enlargement, most frequently seen in deep areas of tumor (Figs. 2.38, 2.39, and 2.40).
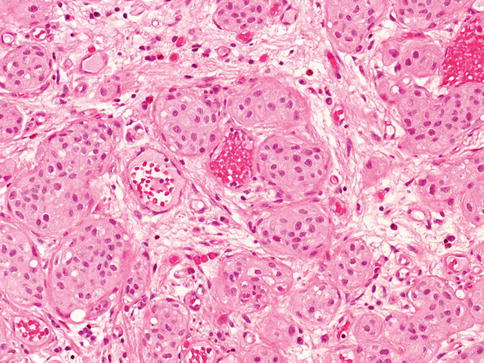
Fig. 2.38
Microscopic features of nested type of urothelial carcinoma
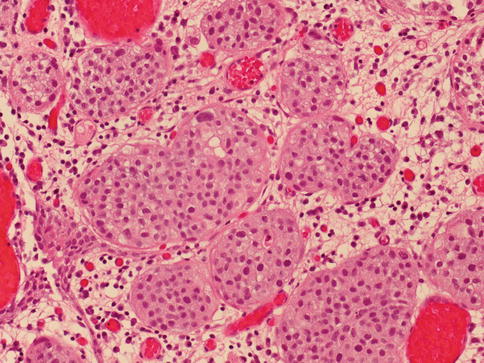
Fig. 2.39
Microscopic features of nested type of urothelial carcinoma, deeper aspect
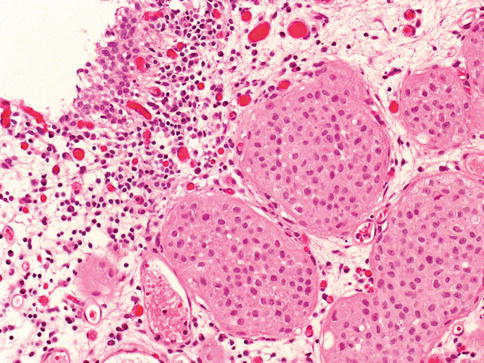
Fig. 2.40
Microscopic features of large nested urothelial carcinoma
Nuclei generally show little or no atypia, but invariably the tumor contains foci of unequivocal cancer with cells exhibiting enlarged nucleoli and coarse nuclear chromatin. Anaplastic features are often more apparent in the deeper aspects of the cancer. The nested urothelial carcinoma, seen either in pure form or with a component of usual urothelial carcinoma, had poor outcomes.
The architectural pattern of nested carcinoma component ranged from a predominantly disorderly proliferation of discrete, small, variably sized nests to tubular growth pattern with focal random nuclear atypia centered within the base of the tumor. A component of usual urothelial carcinoma is frequently present.
The differential diagnosis of the nested variant of urothelial carcinoma include lesions and tumors with nested-like morphology as prominent von Brunn’s nests, cystitis cystica, cystitis glandularis, inverted papilloma, nephrogenic metaplasia, carcinoid tumor, paraganglionic tissue, and paraganglioma.
The immunohistochemical features of the nested variant of urothelial carcinoma are similar to those of aggressive urothelial carcinomas of the usual type, with frequent loss of p27 and a high MIB-1 labeling index. In the differential diagnosis between florid von Brunn nests and the nested variant of urothelial carcinoma, CK20 immunohistochemical evaluation does not appear to be useful, but significantly greater MIB-1 expression and p53 expression are seen in nested variant urothelial carcinoma when compared to florid von Brunn nests, with MIB-1 expression in >7 % of lesional cells and p53 expression in >3 % of lesional cells seen only in carcinoma.
The nested component expressed cytokeratins 7, 20, CAM5.2, and high molecular weight (34ßE12), p63, Ki67, p53, p27, and GATA3. Tumor extension ranged T1 to T4a. On follow-up, 36 of patients died of or were alive with disease from 2 to 80 months (mean 21 months).
Univariate survival analysis showed no differences for nested carcinoma compared with conventional urothelial carcinoma. As in conventional urothelial carcinoma, in nested carcinoma of the bladder pT category defined different survival groups.
Urothelial carcinoma, inverted growth (endophytic) variant
This variant also has been referred as urothelial carcinoma with endophytic (inverted papilloma-like) growth or inverted urothelial carcinoma. The potential for misinterpretation of urothelial carcinoma with inverted growth as benign inverted papilloma is high (Table 2.14).
Table 2.14
Differences between urothelial carcinoma with inverted growth and inverted papilloma
Characteristics
Urothelial carcinoma, inverted growth
Inverted papilloma
Surface
Variable, usually exophytic papillary lesion present
Smooth, dome shaped, usually intact cytologically unremarkable surface urothelium
Growth pattern
Endophytic, thick trabeculae, circumscription variable
Endophytic, sharply delineated, anastomosing cords and trabeculae
Cytologic features
Cytologic atypia is invariably present, mitotic figures often present, less maturation or palisading
Orderly polarized cells, some spindling and palisading at periphery, absence of necrosis and diffuse severe cytologic atypia. Mitotic figures absent or rare
Biologic potential
Recurrences and progression may occur
Benign, no recurrences when completely resecteda
Immunohistochemistry
Variable with grade; usually high p53 accumulation or Ki67-MIB1 counts
Low p53 accumulation and Ki-67-MIB1 counts. 45 % FGFR3 mutations. No TP53 mutations
UroVision FISH
Positive
Negative
The inverted variant of urothelial carcinoma demonstrates significant nuclear pleomorphism, architectural abnormality and increased mitotic activity. In most cases, the surface of the neoplasm shows similar abnormalities and is readily recognized as typical urothelial carcinoma.
An exophytic papillary or invasive component is often associated with the inverted element. However, in cases of inverted papilloma fragmented during transurethral resection, a pseudoexophytic pattern may result. Large papillary tumors with prominent endophytic growth may appear to “invade” the lamina propria with a pushing border. Unless this pattern is accompanied by true destructive stromal invasion the likelihood of metastasis is minimal, because the basement membrane is not truly breached.
A recent proposal suggests grading these tumors following the same criteria as usual urothelial carcinoma (Figs. 2.41 and 2.42).
Fig. 2.41
Microscopic features of low grade urothelial carcinoma with inverted growth
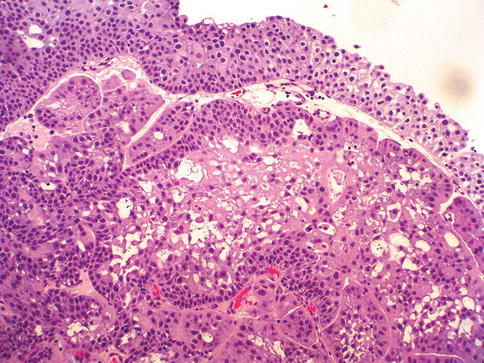
Fig. 2.42
Microscopic features of high grade urothelial carcinoma with inverted growth
Inverted papillomas of the urinary bladder and urothelial carcinomas with an inverted growth pattern may be distinguished using a combination of morphologic, immunohistochemical, and molecular genetic assessments.
Whereas inverted papillomas usually do not demonstrate immunoreactivity for Ki-67, p53, or cytokeratin 20, urothelial carcinomas with inverted growth pattern frequently express one or more of these biomarkers. Similarly, inverted papillomas do not show the molecular features of urothelial carcinoma on UroVysion FISH analysis, whereas inverted-pattern urothelial carcinomas often demonstrated genetic alterations that are commonly seen in bladder cancer.
Urothelial carcinoma, micropapillary variant
Micropapillary carcinoma is a distinct variant of urothelial carcinoma that resembles papillary serous carcinoma of the ovary. Approximately 60 cases have been reported in the literature.
There is a male predominance and the patient age ranges from the fifth to the ninth decade with a mean age of 66 years. Micropapillary carcinoma should be considered a variant of urothelial carcinoma with poor prognosis.
As recently reported, 62 % of patients with invasive Micropapillary carcinoma had lymph node metastases, with 85 % of patients died of disease at a mean interval from the diagnosis of 6.2 months.
A recent study by the MDACC suggested that high grade urothelial carcinoma stage pT1 has a high frequency of micropapillary carcinoma (so called superficial micropapillary carcinoma) and that patients in this category should be offered aggressive therapy instead of intravesical immunotherapy to improve long term survival.
Some studies suggest that the prognosis is related to the proportion of the micropapillary component. Cases with a moderate or extensive micropapillary component are at high risk for having an advanced stage at presentation. Cases with less than 10 % micropapillary component and a surface micropapillary component have a high chance of detection at an early stage.
Micropapillary carcinoma is accompanied by noninvasive papillary or invasive urothelial carcinoma in 80 % of reported cases. The presence of a micropapillary component at the surface in bladder biopsy specimens is an unfavorable prognostic feature. In such cases deeper biopsies may prove useful, since muscle invasion is a significant concern in these cases.
Micropapillary carcinoma is composed of infiltrating slender delicate filiform processes or small tight papillary tumor cell clusters that lie within lacunae that resemble lymphovascular spaces; however, no lining endothelial cells are demonstrable by immunohistochemistry in these small lacunar spaces.
Interobserver reproducibility for a diagnosis of IMPC remains moderate (kappa: 0.54). Although classification as IMPC among the 10 “classic” IMPC cases was relatively uniform (93 % agreement), the classification in the subset of 20 invasive urothelial carcinomas with extensive retraction and varying sized tumor nests was more variable. Multiple nests within the same lacunar space had the highest association with a diagnosis of classic IMPC (Figs. 2.43, 2.44, 2.45, and 2.46).
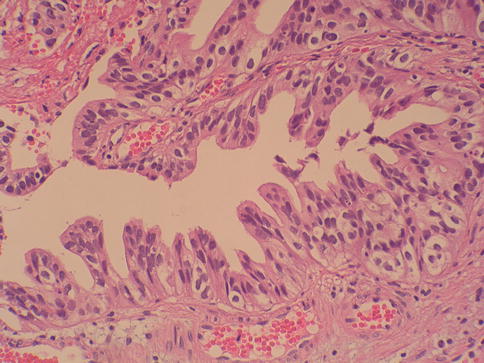
Fig. 2.43
Microscopic features of early micropapillary (superficial) urothelial carcinoma
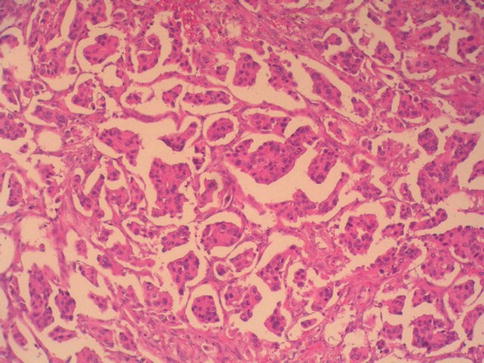
Fig. 2.44
Microscopic features of invasive micropapillary urothelial carcinoma (low power)
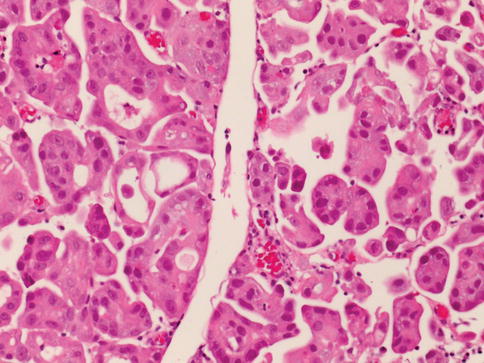
Fig. 2.45
Microscopic features of invasive micropapillary urothelial carcinoma with focal ring formation
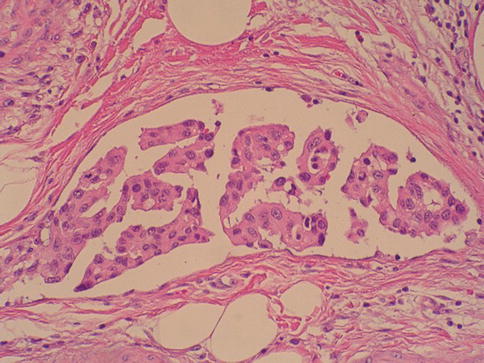
Fig. 2.46
Microscopic features of invasive micropapillary urothelial carcinoma with small papillae within lacunae
Twenty-five percent of cases show glandular differentiation, and some authors consider it a variant of adenocarcinoma. Psammoma bodies are infrequent. True vascular and lymphatic invasion is commonly demonstrable, and most cases show invasion of the muscularis propria or deeper.
Metastases are common at the time of initial diagnosis. The main differential consideration is metastatic serous micropapillary ovarian carcinoma in women or mesothelioma in either gender.
Expression of keratins by tumor cells of micropapillary carcinoma is similar to that of typical urothelial carcinoma, but micropapillary carcinomas are much more likely to express CA-125, suggesting that the micropapillary phenotype is a form of glandular differentiation. Micropapillary carcinoma also show positive immunostaining for EMA, cytokeratins 7 and 20, and Leu M-1 (CD-15). MUC1 expression is seen in the stroma-facing aspect of the tumor cell groups, indicating a reversal of the normal cell orientation in these tumors.
ERBB2 amplification by FISH technology is more frequently observed in micropapillary urothelial carcinoma than in conventional urothelial carcinoma. These patients have worse cancer-specific survival. It also provides a role for ERBB2 targeted therapy.
Carcinomas with micropapillary histology have also been reported in the lung, breast, pancreas, colo-rectum, and salivary glands. Clinical correlation is usually required, but the possibility of a bladder primary may be suggested if there is no obvious primary tumor at another anatomic site. Identification of an admixed urothelial carcinoma of more typical morphology or immunohistochemical support (CK7, CK20, and uroplakin III positivity) would be helpful.
Urothelial carcinoma, microcystic variant
The microcystic variant of invasive urothelial carcinoma is characterized by microcysts, macrocysts, or tubular structures with cysts ranging from microscopic up to 2 cm in diameter (Fig. 2.47).
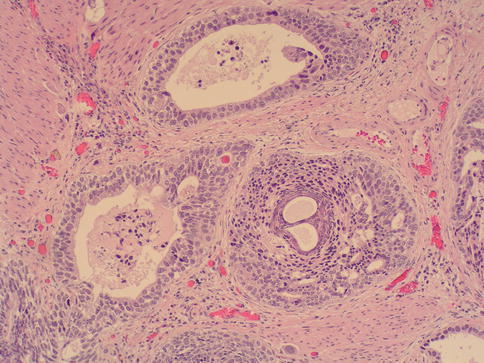
Fig. 2.47
Microscopic features of invasive microcystic carcinoma of the bladder
A recent report based on the clinicopathological features of 20 cases of microcystic urothelial bladder carcinoma showed the cysts were round–oval and of varying sizes; the periphery of large cysts was frequently punctuated by many smaller cysts. The cysts were lined by urothelial, low columnar cells or by a single layer of flattened epithelium of low–intermediate nuclear grade. Focal high-grade conventional urothelial carcinoma was present in eight cases.
On follow-up, 60 % of patients died of disease at 11–56 months. Univariate survival analysis showed no differences for microcystic carcinoma versus conventional urothelial carcinoma.
This variant of urothelial carcinoma may be confused with benign proliferations such as florid polypoid cystitis cystica and glandularis and nephrogenic metaplasia.
This pattern should be separated from the nested variant of urothelial carcinoma with tubular differentiation.
Rarely, microcystic variant may simulate Gleason 3 + 3 prostatic adenocarcinoma showing negative prostate markers and positive for urothelial markers (CK7, CK20 and thrombomodulin).
Immunohistochemistry demonstrated variable positivity for cytokeratins 7 and 20, MUC1, MUC5AC, p63 and GATA3. Extent of expression of Ki67, p53 and p27kip1 ranged from 20 to 60 %, 10–40 % and 10–30 % of cells, respectively.
Lymphoepithelioma-like (urothelial) carcinoma
Carcinoma that histologically resembles lymphoepithelioma of the nasopharynx has been described in the urinary bladder, with fewer than 100 cases reported. It is more common in men than in women (3:1 ratio) and tends to occur in late adulthood (range: 52–81 years; mean: 69 years).
Most patients present with hematuria. The tumor is solitary and usually involves the dome, posterior wall, or trigone, often with a sessile growth pattern. Histologically, it may be pure or mixed with typical urothelial carcinoma, the latter being focal and inconspicuous in some instances.
Glandular and squamous differentiation may be seen. The epithelial component is composed of nests, sheets, and cords of undifferentiated cells with large pleomorphic nuclei and prominent nucleoli (Fig. 2.48). The cytoplasmic borders are poorly defined, imparting a syncytial appearance. The background consists of a prominent lymphoid stroma that includes T and B lymphocytes, plasma cells, histiocytes, and occasional neutrophils or eosinophils.
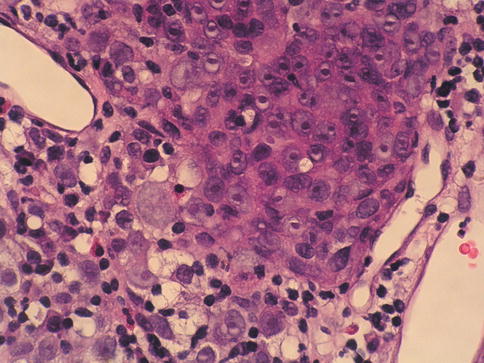
Fig. 2.48
Lymphoepithelioma-like carcinoma of the urinary bladder showing syncytial arrangement of the cells
Epstein-Barr virus or human papilloma virus infection have not been identified in lymphoepithelioma-like carcinoma of the bladder. Immunohistochemistry reveals cytokeratin immunoreactivity (cytokeratin AE1/3 and cytokeratin 7) in the malignant cells, confirming their epithelial nature. The epithelial cells are rarely positive or negative for cytokeratin 20. Lelc cases have common Urovysion probes alteration and frequent p53 accumulations supporting a similar pathogenesis to conventional urothelial carcinoma.
The major differential diagnostic considerations are poorly differentiated urothelial carcinoma with lymphoid inflammatory response, poorly differentiated squamous cell carcinoma, and lymphoma. Lymphoepithelioma-like bladder carcinoma has been found to be responsive to chemotherapy if the tumor is encountered in its pure form.
Although most reported cases occurring in the urinary bladder are associated with a relatively favorable prognosis when pure or predominant, when lymphoepithelioma-like carcinoma is only focally present in an otherwise typical urothelial carcinoma, the prognosis is the same as that of patients with conventional urothelial carcinoma of the same grade and stage.
Urothelial carcinoma, plamacytoid variant
It is composed of cells with a monotonous appearance mimicking lymphoma. The tumor cells were medium-sized, with eosinophilic cytoplasm and eccentric nuclei producing a plasmacytoid appearance. The differential diagnostic considerations include lymphoid reaction, lymphoma, multiple myeloma, urothelial carcinoma with rhabdoid feature, signet ring cell adenocarcinoma, paraganglioma, neuroendocrine carcinoma, melanoma, and rhabdomyosarcoma.
Identification of an epithelial component by Immunohistochemistry confirms the diagnosis. A series report of 11 cases showed that the plasmacytoid component comprised greater than 50 % of the tumor in eight cases, with two additional cases showing pure plasmacytoid carcinoma (Figs. 2.49, 2.50, and 2.51).
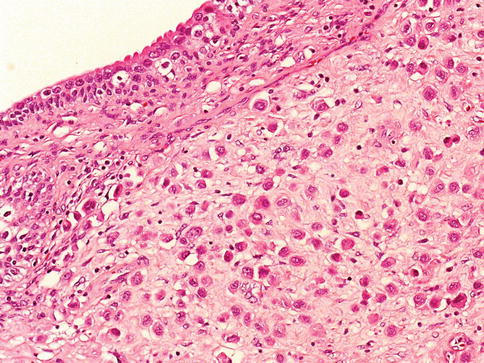
Fig. 2.49
Microscopic features of plasmacytoid urothelial carcinoma
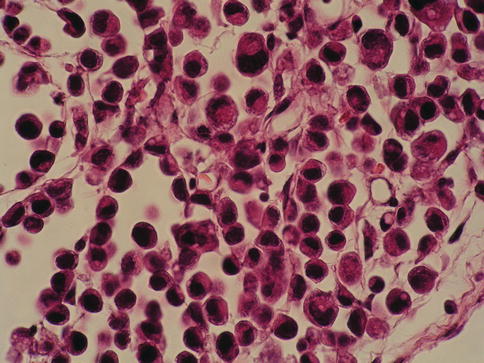
Fig. 2.50
High power view of plasmacytoid urothelial carcinoma
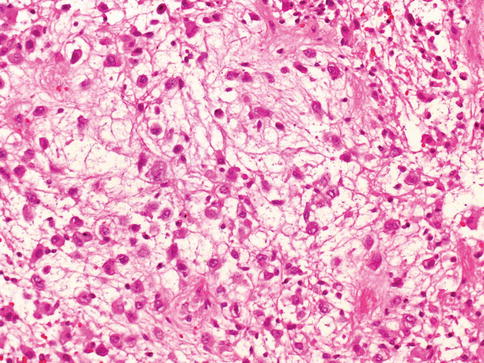
Fig. 2.51
Occasionally, plasmacytoid carcinoma shows myxoid stroma
All patients had advanced-stage cancer (>pT3), and 73 % had lymph node metastases. On follow-up, 82 % of patients died of disease from 2 to 11 months, and two patients were alive with disease at 8 and 16 months.
The architectural pattern of the tumor varied from solid expansile nests with discohesive cells to mixed solid and alveolar growth; a streaking discohesive architecture was additionally present in two cases (18 %).
Rarely, a myxoid pattern can be present. Histologically, the individual tumor cells had an eccentrically placed nucleus and abundant eosinophilic cytoplasm reminiscent of plasma cells. Most neoplastic cells had nuclei of low to intermediate nuclear grade with occasional nuclear pleomorphism. Small intracytoplasmic vacuoles were variably present in all cases.
Immunohistochemical staining demonstrated that both plasmacytoid and associated conventional urothelial carcinoma were positive for CK7, CK20, AE1/AE3, and EMA; CD138 (marker of plasma cells) was positive in three cases. Ring-like structures are most consistent features in diagnosis.
Artefactual expression of kappa light chains have been seen focally is some cases, a further confounding factor.
Dyscohesive growth pattern and morphological features mimicking infiltrating lobular carcinoma of the breast and diffuse carcinoma of the stomach have been described in some cases, more frequently male patients with the mean age of 67
All the cases showed single-cell diffusely infiltrative growth pattern. In some areas the tumor cells were arranged in linear single-cell files (Indian-file pattern) and in other areas there were solid sheets of dyscohesive cells. In all cases there were tumor cells showing prominent intracytoplasmic vacuoles. Half of reported cases also showed typical transitional cell carcinoma or carcinoma in situ.
The tumor cells expressed cytokeratin 20 but not estrogen receptor, and some were negative for E-Cadherin. Currently, most authorities place discohesive growth pattern as a form of plasmacytoid variant of bladder cancer.
Urothelial carcinoma, clear cell (glycogen-rich) variant
Up to two-thirds of urothelial carcinoma cases have foci of clear cell change resulting from abundant glycogen. The glycogen-rich clear cell “variant” of urothelial carcinoma, recently described, appears to represent the extreme end of the morphologic spectrum.
It consists predominantly or exclusively of cells with abundant clear cytoplasm (Fig. 2.52). Tumor cells show positive immunostaining for cytokeratin 7.
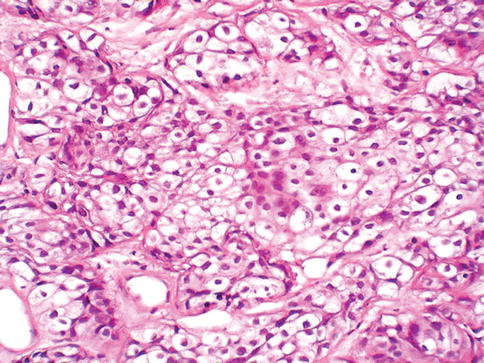
Fig. 2.52
Urothelial carcinoma with glycogen-type clear cells
Recognition of this pattern avoids confusion with clear cell adenocarcinoma of the bladder and metastatic clear cell carcinoma from the kidney or prostate. Cytoplasmic clearing as a result of thermal artifact in transurethral resections should not be mistaken for this variant of bladder cancer.
Urothelial carcinoma, lipid cell variant
Lipid cell variant is a rare neoplasm defined as an urothelial carcinoma which exhibits transition to a cell type resembling signet-ring lipoblasts (Figs. 2.53 and 2.54).
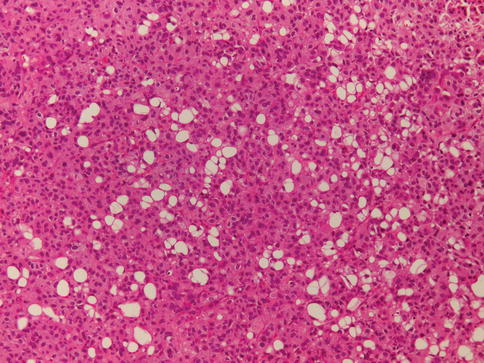
Fig. 2.53
Lipid variant of urothelial carcinoma (low power)
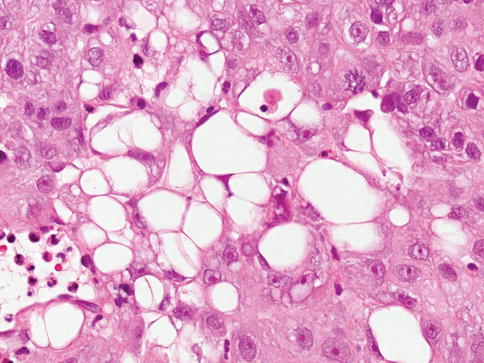
Fig. 2.54
Lipid variant of urothelial carcinoma (high power)
A recent report based on 27 cases showed that the lipid cell component varied from 10 to 50 % of the tumor specimen. Pathologic stage at diagnosis was Ta (n = 1), T1 (n = 2), T2, (at least n = 7), T3a (n = 4), T3b (n = 8), and T4a (n = 5). Sixteen of the patients died of disease from 16 to 58 months (mean, 33 months).
The architectural pattern of the tumor varied from solid expansile to infiltrative nests. The large epithelial tumor cells had an eccentrically placed nucleus and abundant vacuolated cytoplasm resembling signet ring lipoblasts. Mucin stains were negative in all cases. Most neoplastic cells had nuclei of intermediate nuclear grade with occasional nuclear pleomorphism.
Immunohistochemical staining demonstrated that the lipid cell component was positive for cytokeratins 7, 20, CAM5.2, high-molecular-weight (34βE12) and AE1/AE3, epithelial membrane antigen, and thrombomodulin; vimentin and S-100 protein were negative. The loss of heterozygosity (LOH) analysis was performed on eight cases. The LOH results were the same for lipid variant and concurrent conventional urothelial carcinoma.
Electron microcopy analysis based on two cases supported lipid content in tumor cells. In limited samples, lipid cell variant urothelial carcinoma may be misdiagnosed as liposarcoma, sarcomatoid carcinoma (carcinosarcoma) with a liposarcomatous component, or signet ring cell carcinoma. The finding of lipid cells immunoreactive for epithelial markers can be very useful in this setting.
Urothelial carcinoma with syncytiotrophoblastic giant cells
Syncytiotrophoblastic giant cells are present in up to 28 % of urothelial carcinoma cases, producing substantial amounts of immunoreactive beta-human chorionic gonadotropin (HCG) indicative of syncytiotrophoblastic differentiation (Fig. 2.55).
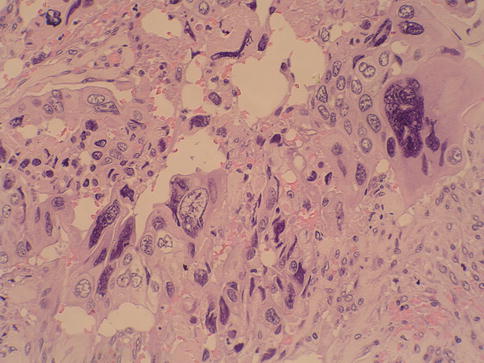
Fig. 2.55
Urothelial carcinoma with trophoblastic differentiation
The number of HCG-immunoreactive cells is inversely associated with cancer grade. Secretion of HCG into the serum may be associated with a poor response to radiation therapy.
The most important differential diagnostic consideration is choriocarcinoma whose diagnosis may require a high copy number of the isochromosome 12p as seen by FISH, thus supporting germ cell differentiation. HCG expression in poorly differentiated urothelial carcinoma without overt syncytiotrophoblastic differentiation probably represents a metaplastic phenomenon. It seems likely previously reported “primary choriocarcinomas of the bladder” represent urothelial carcinoma with syncytiotrophoblasts.
Urothelial Carcinoma with Small Tubules
Although a prominent tubular component may accompany a nested carcinoma, some urothelial carcinomas may have an almost exclusive component of small- to medium-sized, round to elongated tubules that may be misdiagnosed as nephrogenic adenoma, adenocarcinoma, or cystitis glandularis (Fig. 2.56).
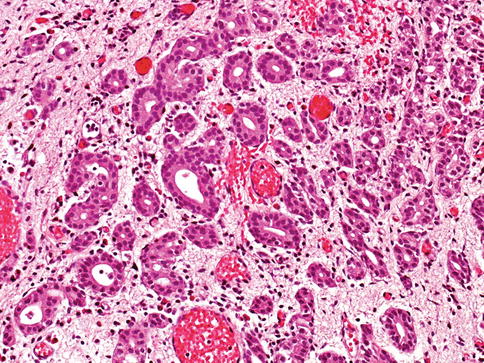
Fig. 2.56
Urothelial carcinoma with small tubules lined by attenuated urothelial cells
Furthermore, the tubules of urothelial carcinoma are lined by attenuated urothelial cells, in contrast to the varying admixture of cuboidal, columnar, and occasionally flattened cells that line the tubules of nephrogenic adenoma. Urothelial carcinoma with small tubules may be widely invasive despite their deceptively bland histology.
The significance of this pattern is unclear given the rarity of cases, but some of these cases occur in conjunction with the nested pattern and are widely invasive and have an aggressive outcome because of their high stage at presentation.
Similar to the nested variant, the chief reason for the awareness of this morphological variant of urothelial carcinoma is not to mistake it in superficial biopsies as a benign glandular proliferative lesion. Some authors believe that urothelial carcinoma with small tubules should be considered within the spectrum of nested carcinoma.
The differential diagnosis with an extension of a prostatic carcinoma is often also a consideration but is easily handled by immunohistochemistry prostate-specific antigen (PSA), prostatic-specific acid phosphatase (PSAP): positive in prostate cancer; CK20, high-molecular-weight cytokeratin; and p63: positive in more than half of urothelial carcinomas.
Urothelial Carcinoma with myxoid stroma
This is a recently described entity with myxoid stroma and chordoid-like morphology characterized by prominent cellular cording and associated myxoid stromal matrix, a pattern that may resemble extraskeletal myxoid chondrosarcoma (Figs. 2.57 and 2.58).
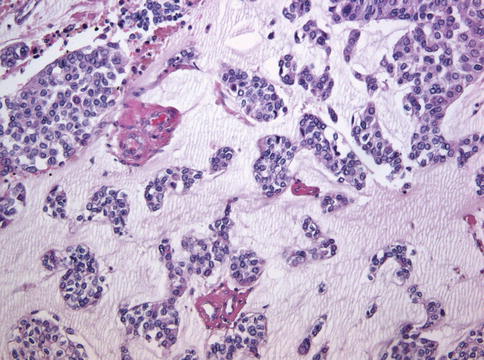
Fig. 2.57
Urothelial carcinoma with myxoid stroma showing a mucinous appearance
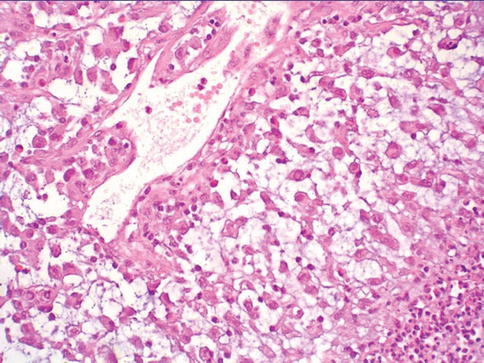
Fig. 2.58
Urothelial carcinoma with myxoid stroma showing a chordoid-like pattern
These carcinomas maintain an immunophenotype characteristic of urothelial carcinoma and usually present with high-stage disease. The patients’ ages ranged from 50 to 85 years (mean: 68 years); there were eight males and four females. Morphologically, each case had at least focal areas in which acellular myxoid stroma was associated with the carcinoma cells.
Occasionally, cells were arranged into cords closely mimicking extraskeletal myxoid chondrosarcoma, chordoma, mixed tumor/myoepithelioma of soft tissue, and yolk sac tumor. All 12 cases had foci of typical urothelial carcinoma present at least focally.
Immunophenotypically, these tumors show strong immunoreactivity for p63 (nuclear) and CK34βE12 (cytoplasmic).
Immunostains for CK20, calponin, glial fibrillary acidic protein, oncofetal protein glypican-3, and brachyury and were negative in the seven cases studied, whereas S-100 protein had focal staining (<5 %) in one case. The myxoid stromal component was diffusely colloidal iron and Alcian blue positive; periodic acid–Schiff was negative in all eight cases, whereas mucicarmine was focally positive in only two of eight cases. Most cases were high stage and 75 % with nodal sampling had metastatic disease.
Nine of 12 patients had available follow-up: two were dead of disease (1 and 10 months), four were alive with disease (5–8 months) with distant metastasis in three, and three had no evidence of disease at last follow-up (2–120 months).
Sarcomatoid carcinoma
The term sarcomatoid carcinoma applies when a malignant neoplasm exhibits morphologic or immunohistochemical evidence of both epithelial and mesenchymal differentiation. Heterologous elements may be present and should be acknowledged in the pathology report. We postulate that sarcomatoid carcinoma is a common final pathway of all forms of epithelial bladder tumors, a hypothesis supported by molecular data and morphogic evidence (Figs. 2.59, 2.60, and 2.61).
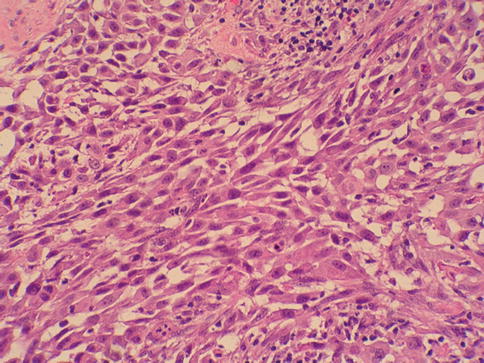
Fig. 2.59
Sarcomatoid carcinoma with atypical spindle cells
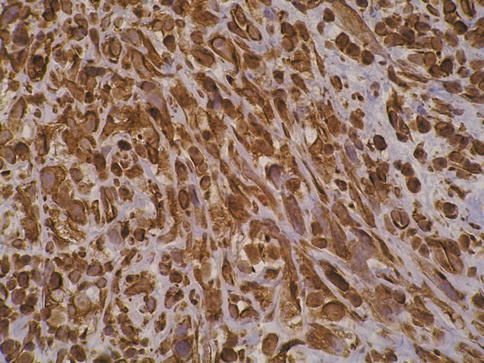
Fig. 2.60
Spindle cells in sarcomatoid carcinoma are positive for cytokeratin
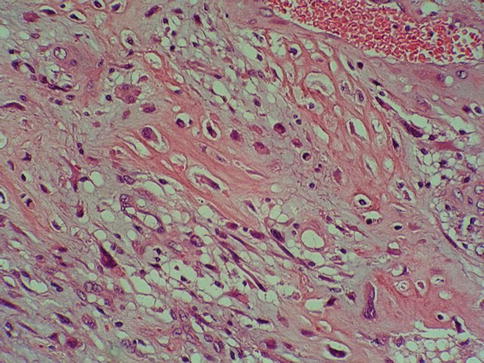
Fig. 2.61
Sarcomatoid carcinoma with heterologous (osteosarcoma) differentiation
Sarcomatoid transformation has been observed in various epithelial tumor of the urinary bladder, including conventional urothelial carcinoma, small cell carcinoma, adenocarcinoma, squamous cell carcinoma, large cell neuroendocrine carcinoma.
Various terms have been used for these neoplasms, including carcinosarcoma, sarcomatoid carcinoma, pseudosarcomatous transitional cell carcinoma, malignant mesodermal mixed tumor, spindle cell carcinoma, giant cell carcinoma, and malignant teratoma.
In some reports, both carcinosarcoma and sarcomatoid carcinoma are included under the term “sarcomatoid carcinoma”. In others they are regarded as separate entities. There is widespread consensus that the most appropriate term for all of these neoplasms is sarcomatoid carcinoma. We prefer to use the term sarcomatoid carcinoma for the majority of cases in which there is a spindle cell component with positive vimentin staining. Some may use the term carcinosarcoma for cases with identifiable heterologous elements on hematoxylin and eosin stained sections or positive staining for markers of specific mesenchymal differentiation.
Both diagnostic categories appear to be variations of the same neoplastic transformation process and have the same clinical features and prognosis.
Sarcomatoid carcinoma represents approximately 0.3 % of bladder carcinomas. The most frequent presenting symptoms are hematuria, dysuria, nocturia, acute urinary retention, and lower abdominal pain. The mean patient age is 66 years (range 50–77 years). In some patients, there is a history of carcinoma treated by radiation, or exposure to cyclophosphamide therapy.
The presence of specific types of differentiation of the spindle cell component does not influence the prognosis, but sarcomatoid carcinoma is usually high grade, biologically aggressive and associated with a poor prognosis. Pathological stage is the best predictor of survival in sarcomatoid carcinoma.
Pathology
The gross appearance is characteristically “sarcoma-like,” with a dull gray fleshy cut surface and infiltrative margins. The tumors are often polypoid and tend to form large intraluminal masses. Microscopically, sarcomatoid carcinoma is composed of a urothelial, glandular or small cell epithelial component showing variable degrees of differentiation.
Carcinoma in situ is present in 30 % of cases and occasionally is the only apparent epithelial component. A small subset of sarcomatoid carcinomas may have a prominent myxoid stroma.
The mesenchymal component most frequently observed is an undifferentiated high grade spindle cell neoplasm. The most common heterologous element is osteosarcoma, followed by chondrosarcoma, rhabdomyosarcoma, leiomyosarcoma, liposarcoma, angiosarcoma or multiple types of mesenchymal differentiation.
Differential diagnosis and immunohistochemistry
Sarcomatoid carcinoma is characterized by strong staining with keratins (AE1/AE3, CAM5.2) and/or epithelial membrane antigen with coexpression of vimentin in the majority of cases. In contrast to smooth muscle neoplasms, actin and desmin are typically negative. However, sarcomatoid carcinomas with heterologous differentiation may rarely be encountered and in this situation expression of other mesenchymal markers such as actin, desmin, S-100 protein may be observed.
Sarcomatoid carcinoma of the urinary bladder is usually biphasic, composed of both epithelial and mesenchymal elements. In cases exclusively composed of spindle cells, the main differential diagnostic consideration is sarcoma, particularly leiomyosarcoma. In view of the rarity of primary bladder sarcoma, any malignant spindle cell tumor in the urinary bladder in an adult be considered sarcomatoid carcinoma until proven otherwise.
Cytokeratin immunostaining may be helpful in this setting. One should also bear in mind that focal cytokeratin immunoreactivity may be seen in smooth muscle tumors and pseudosarcomatous myofibroblastic proliferations. Smooth muscle tumors are additionally immunoreactive for desmin and vimentin, even when they express cytokeratin antigens.
Sarcomatoid carcinoma with prominent myxoid and sclerosing stroma may be mistaken for pseudosarcomatous myofibroblastic tumor, postoperative spindle cell nodule, or urothelial carcinoma with pseudosarcomatous stroma. The presence of slit-like vessels and the absence of mitotic figures and significant cytologic atypia favor the diagnosis of benign lesion.
Molecular genetics
Recent molecular studies strongly argue for a monoclonal origin of both the epithelial and mesenchymal components in sarcomatoid carcinoma and carcinosarcoma. These two categories have similar clinical characteristics, including patient age, gender presentation and outcome.
A recent analysis of 30 sarcomatoid urothelial carcinomas for X-chromosome inactivation status as well as loss of heterozygosity (LOH) has shed light on the pathogenesis of this tumor. Results of this study found identical patterns of allelic loss in the carcinomatous and in the sarcomatous components, identified at four of six polymorphic microsatellite markers where genetic alterations occur frequently in urothelial carcinomas, including D8S261 (86 %), D9S177 (75 %), IFNA (57 %), and D11S569 (78 %) loci.
Furthermore, discordant allelic loss of microsatellite markers was also found at the various sites as the bladder tumors underwent malignant progression leading to genetic divergence of high grade anaplastic malignancies following the initial neoplastic transformation. Additionally, the study found the same pattern of nonrandom X-chromosome inactivation in both carcinomatous and sarcomatous components in 5 of 8 female patients.
The identical X-chromosome inactivation and significant concordance of LOH support the contention that both carcinomatous and sarcomatous tumor components arise from a monoclonal primordial cell. Clonal divergence may occur during tumor progression and lead to differentiation into mesenchymal as well as epithelial phenotypes.
Small cell carcinoma
Small cell carcinoma is a malignant neoplasm derived from the urothelium which mimics its pulmonary counterpart (discussed later in this chapter, page ). It often co-exists with conventional urothelial carcinoma, squamous carcinoma, and adenocarcinoma or sarcomatoid carcinoma.
Urothelial carcinoma with rhabdoid features
A variety of tumors with rhabdoid differentiation have been described in the literature. Very few cases have been described in the urinary tract, mainly in the bladder.
In all cases, the rhabdoid component was admixed with other components, including conventional urothelial carcinoma, sarcomatoid carcinoma, and/or small cell carcinoma.
Rhabdoid cells have abundant eosinophilic cytoplasm, vesicular nuclei, and prominent nucleoli. they show positive immunostaining for cytokeratin markers (epithelial membrane antigen, CAM5.2, and AE1/3) (Figs. 2.62 and 2.63). These tumors are highly aggressive: 50 % of patients die of bladder cancer shortly after the diagnosis.
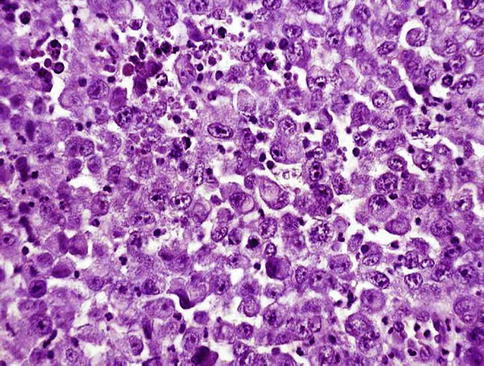
Fig. 2.62
Microscopic features of rhabdoid tumor of the urinary bladder
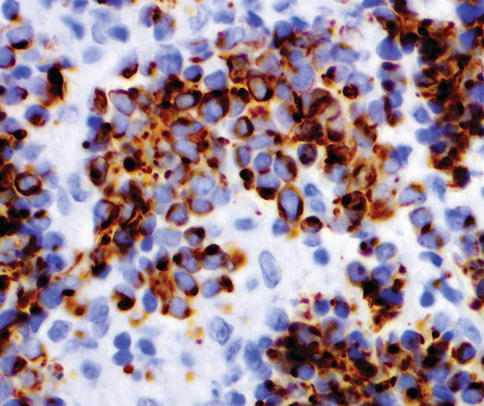
Fig. 2.63
Rhabdoid tumor cells express cytokeratin in a dot-like manner< div class='tao-gold-member'>Only gold members can continue reading. Log In or Register to continue
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree








