, Carmen L. Menendez2, Rodolfo Montironi3 and Liang Cheng4
(1)
Department of Surgery and Pathology, University of Cordoba Faculty of Medicine, Cordoba, Spain
(2)
Pathology Department, Hospital de Cabueñes, Gijón, Spain
(3)
Department of Biomedical Sciences and Public Health, Polytechnic University of the Marche Region (Ancona), Torrette, Italy
(4)
Department of Pathology, Indiana University School of Medicine, Indianapolis, Indiana, USA
5.6 Secondary Tumors
5.8 Other Carcinomas
5.10 Soft Tissue Tumors
5.11 Other Rare Tumors
5.12 Secondary Tumors
5.1 Basic Anatomy and Histology
5.1.1 Penis
Penile anatomical regions are glans, foreskin, and shaft. Glans is distal, formed by corpus spongiosum covered by squamous mucosa. Distal urethra opens up into meatus, a ventrally located slit-like orifice in glans.
Glans corona separates glans from coronal sulcus. Coronal sulcus is cul-de-sac between glans and foreskin. Frenulum connects foreskin to ventral portion of glans corona. Foreskin covers glans and presents mucosal (inner) and cutaneous (outer) surface.
Penile root anchors penis to perineal membrane and pubic arc. Penile shaft is composed mainly by ventral column of corpus spongiosum and two dorsal columns of corpora cavernosa.
Glans, coronal sulcus, and inner foreskin are covered by nonkeratinized squamous epithelium overlying loose lamina propria.
Penile erectile tissues comprising two corpora cavernosa and corpus spengiosum surrounding penile urethra form body of penile shaft.
Irregular vascular spaces with intermingling elastic connective tissue form penile erectile tissues. Vascular spaces of corpus spongiosum are more widely spaced and irregular when compared with corpus cavernosum.
Corpus spongiosum is also covered by tunica albuginea. Tunica albuginea composed of dense connective tissue encompasses both corpus cavernosus and separates them from corpus spongiosum.
Outer foreskin and shaft are covered by skin. Bundles of dartos muscle extend underneath dermis throughout shaft and foreskin.
5.1.2 Scrotum
Contains the testes and the lower parts of the spermatic cords. Consists of skin, the dartos muscle, and external spermatic, cremasteric, and internal spermatic fasciae. The internal fascia is loosely attached to the parietal layer of the tunica vaginalis.
The scrotum is divided into right and left halves by a cutaneous raphe which continues ventrally to the inferior penile surface and dorsally along the midline of the perineum to the anus. A median raphe of fibrovascular connective tissue separates the two halves.
Its blood supply derives from the external and internal pudendal arteries. Additional blood comes from the cremasteric and testicular arteries that traverse the spermatic cords. Lymphatic drainage is to the superficial inguinal nodes.
The epidermis covers the dermis. The deepest layer of the dermis merges with the smooth muscle bundles of the dartos tunic. There is no subcutaneous adipose tissue layer, but scattered fat cells are usually present. The dermis contains sebaceous and eccrine, apocrine glands, and hair follicles.
5.2 Carcinoma of the Penis
5.2.1 Overview
Squamous cell carcinoma represents most common malignant tumor of penis. Keratinocytic differentiation is characteristic. Most penile squamous cell carcinomas originate from squamous mucosal surface of distal penis (glans, coronal sulcus, and foreskin). Glans is most common affected site followed by inner foreskin and coronal sulcus. Squamous cell carcinoma of penile shaft is exceedingly rare.
Major risk factors for penile cancer are phimosis and human papillomavirus (HPV) infection (especially by high-risk HPV). History of genital warts, poor hygiene, and smoking is common. Treatment with ultraviolet A (PUVA) therapy is a recently recognized factor.
Thirty to forty percent of all squamous cell carcinomas are HPV-related. High-risk HPV predominates, and HPV16 is most common genotype encountered. HPV18 is second most common genotype. Other reported genotypes include 45, 52, and 74. Low-risk HPV (genotypes 6 and 11) infection is uncommon.
Most frequent in sixth to seventh decades of life. There is wide range of geographical variation with low incidence in USA and Europe and high incidence in South America, Asia, and Africa.
Squamous cell carcinoma of the penis represents approximately 0.5 % of all cancers among men in the United States and other developed countries. Only one-half of patients survive beyond 5 years.
Ulceration may be present but urinary obstruction secondary to urethral tumoral extension is uncommon. About 50 % of penile carcinomas affect multiple anatomic compartments and frequently present as painless tumoral mass.
Patterns of growth include superficial spreading, vertical growth, verruciform, and multicentric, but mixed forms of growth are common.
Superficial spread includes intermediate risk cases with horizontal or superficial extension involving one or more anatomical compartments. The in situ component, usually extensive, shows invasion confined to lamina propria.
Vertical growth is common in deeply infiltrative tumors with frank invasion of corpus spongiosum or corpus cavernosum. Vertical growth tumors show poor outcome and higher rate of nodal involvement.
Verruciform tumors may reach large sizes but tend to be a localized, low metastatic rate and characteristically exophytic, cauliflower-like tumor mass, usually invading only superficial anatomical levels.
Multicentric tumors with presence of two or more independent foci of squamous cell carcinoma are also seen. Each foci should be separately evaluated and reported
Mixed patterns with combinations of any of patterns of growth may be seen.
There is striking correlation of HPV presence and tumor histologic classification. Basaloid and warty (condylomatous) squamous cell carcinoma are HPV related in most cases.
HPV incidence is low in squamous cell carcinoma, usual type, sarcomatoid, and papillary squamous cell carcinoma. Verrucous, pseudohyperplastic, and cuniculatum squamous cell carcinoma are typically HPV negative tumors. Lichen sclerosus and other chronic inflammatory conditions are common in these variants of carcinoma.
Although the diagnosis and classification of penile tumors is straightforward in most cases, a few entities are problematic, especially to pathologists from countries in which penile cancer is rarely encountered.
Pathologic Prognostic Parameters
Over 50 % of the invasive penile squamous cell carcinoma cases are associated with HPV, using p16 (INK4a) as a surrogate marker of HPV infection. These patients had a statistically significant survival advantage, independent of other prognostic factors. The prognosis in high grade cases lacking p16 (INK4a) is worse.
Tumor extension follows local invasion of penile levels with extension to adjacent tissues, scrotum, perineum, prostate and metastasis to inguinal superficial and deep nodes. Metastases to pelvic lymph nodes also occur and when present, this is an adverse prognostic marker, with a 3-year overall survival rate of approximately 12 % (Tables 5.1 and 5.2).
Table 5.1
Prognostic factors for squamous carcinoma of the penis
Grade 3
Perineural invasion
Invasion of corpora cavernosa
Tumor depth or thickness greater than 10 mm
Sarcomatoid and basaloid subtypes
Vertical growth pattern
Table 5.2
Prognostic index score to predict regional nodal involvement and mortality
Histologic grade
Grade 1
1 point
Grade 2
2 point
Grade 3
3 point
Level of infiltration
Level 1 (lamina propria)
1 point
Level 2 (corpus spongiosum-Dartos)
2 point
Level 3 (corpus cavernosum-preputial skin)
3 point
Perineural invasion
Absent
0 point
Present
1 point
Systemic dissemination (nonregional lymph nodes) presents in up to 30 % of patients in high-risk regions. Liver is most common site of metastatic dissemination followed by lungs, heart, and rarely bone involvement (Tables 5.3 and 5.4).
Table 5.3
TNM staging system for penile carcinoma (2010)
Primary tumor (T)
Tx Primary tumor cannot be assessed
T0 No evidence of primary tumor
Tis Carcinoma in situ
Ta Verrucous carcinoma
T1a Tumor invades subepithelial connective tissue. No lymphovascular invasion. No poorly differentiated (No grade 3–4)
T1b Tumor invades subepithelial connective tissue with lymphovascular invasion or is poorly differentiated
T2 Tumor invades corpus spongiosum or cavernosum
T3 Tumor invades urethra
T4 Tumor invades other adjacent structures (perineum, scrotum, psotate)
Pathologic stage (based upon biopsy or surgical excision) (pN)
pNX Regional lymph node cannot be assessed
pN0 No regional lymph node metastasis
pN1 Metastasis in a single inguinal lymph node
pN2 Metastasis in multiple or bilateral lymph nodes
pN3 Extranodal extension of lymph node metastasis or pelvic lymph nodes unilateral or bilateral
Distant metastasis (M)
M0 No distant metastasis
M1 Distant metastasis (lymph node metastasis outside of the true pelvis in addition to visceral or bone sites)
Table 5.4
Pathologic stages/prognostic groups of penile carcinomas
Group
T
N
M
Stage 0
Tis, Ta
N0
M0
Stage I
T1a
N0
M0
Stage II
T1b, T2, T3
N0
M0
Stage IIIa
T1-3
N1
M0
Stage IIIb
T1-3
N2
M0
Stage IV
T4
Any N
M0
Any T
N3
M0
Any T
Any N
M1
Lymphatic or vascular invasion, primary tumor expression of p53, number of metastatic inguinal lymph nodes and lymph node density are predictors of pathologic pelvic lymph node involvement.
5.2.2 Preneoplastic and Other Intraepithelial Lesions
5.2.2.1 Penile Intraepithelial Neoplasia
Penile intraepithelial neoplasia (PeIN) is considered intraepithelial lesion of invasive squamous cell carcinoma (synonyms: Erythroplasia of Queyrat, Bowen disease, squamous cell carcinoma in situ, squamous intraepithelial lesion (SIL)) (Table 5.5).
Table 5.5
Types of penile intraepithelial neoplasia (PeIN)
Differentiated
Undifferentiated
Warty
Warty-Basaloid
Basaloid
Other
Mixed differentiated-undifferentiated
PeIN may present as uni- or multifocal, flat to slightly elevated hyperkeratotic, sharp, or ill-defined borders or even papillary lesions. Clinically seen as moist, white, erythematous, dark brown or black macules, papules, or plaques.
Real incidence is unknown, but most are associated with invasive squamous cell carcinoma, differentiated PeIN (65 %) and undifferentiated PeIN (35 %). Most cases present in fifth and sixth decades.
Differentiated or simplex PeIN is unrelated to HPV. Lichen sclerosus may be implicated in pathogenesis of differentiated PeIN. Frequently affects foreskin.
Warty, warty/basaloid, and basaloid (undifferentiated) PeIN are HPV-related, with frequent HPV16 genotype. Usually affects glans, perimeatal region. Usually not associated with lichen sclerosus. Patients may have history of condyloma or genital warts (Figs. 5.1, 5.2, 5.3, 5.4, 5.5, 5.6, 5.7, 5.8, 5.9 and 5.10).
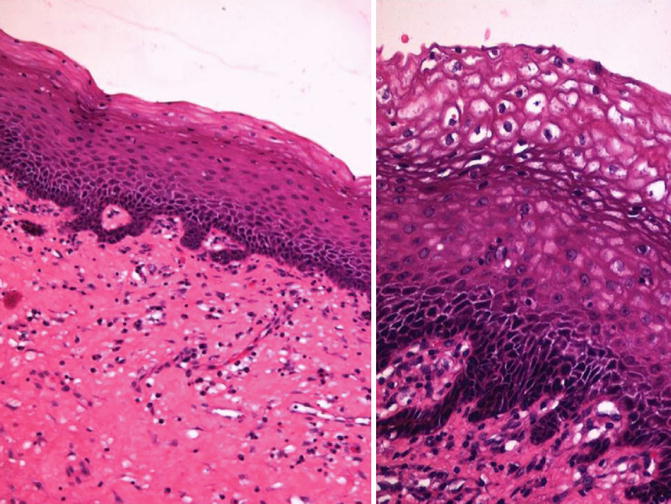
Fig. 5.1
Differentiated-PeIN. Low (left) and high (right) power view with koilocytosis
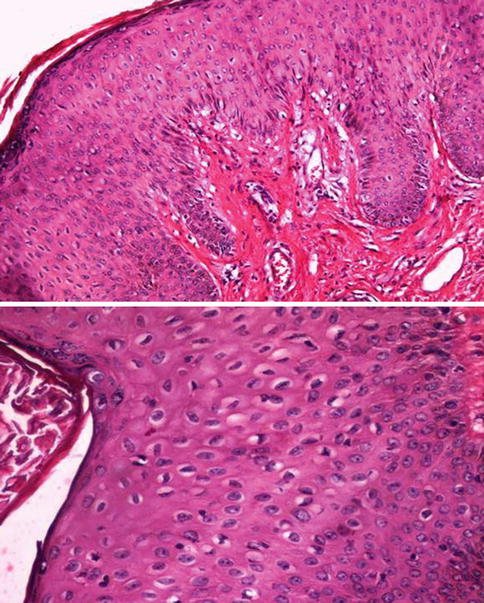
Fig. 5.2
High grade-PeIN (upper) with koilocytosis (lower)
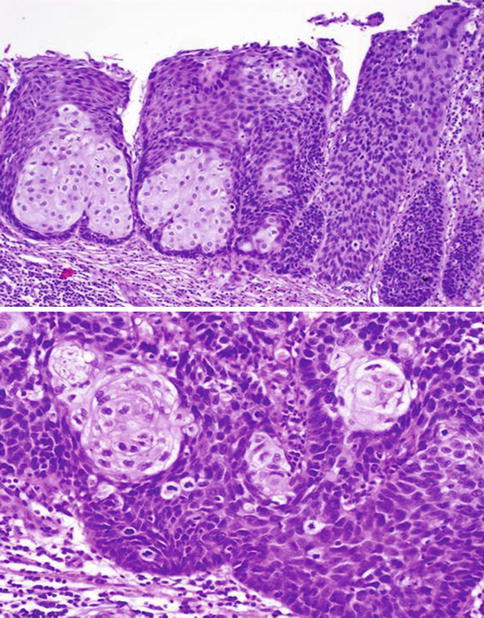
Fig. 5.3
Mixed-basaloid-PeIN. Low (upper) and high (lower) power view
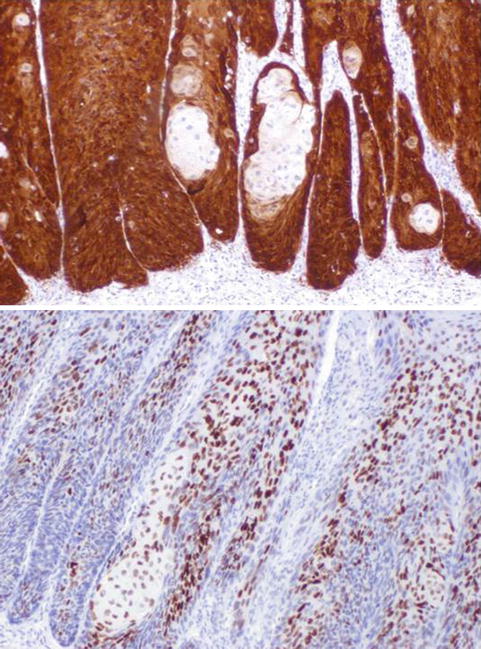
Fig. 5.4
Mixed-basaloid-PeIN. p16 (upper) and p53 (lower)
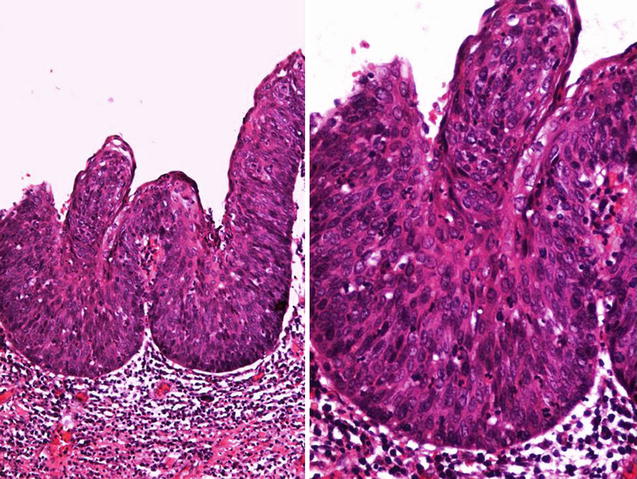
Fig. 5.5
High grade basaloid-PeIN with papillary configuration seen a low (left) and high (right) power
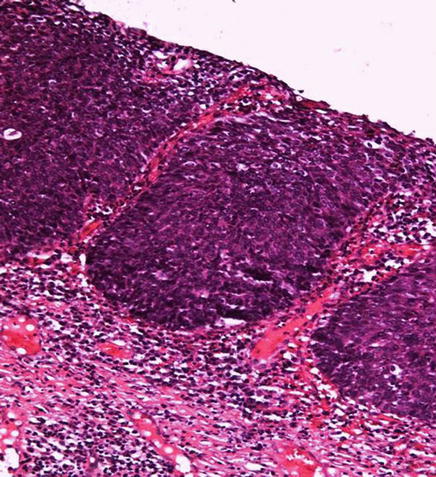
Fig. 5.6
Basaloid-PeIN
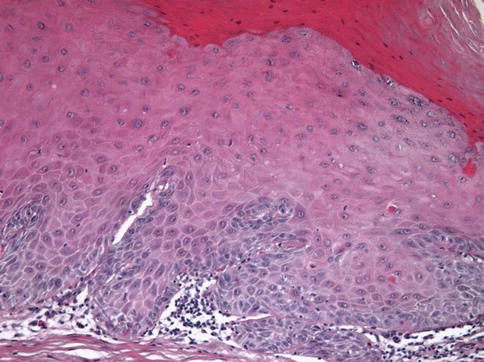
Fig. 5.7
Verrucous-PeIN. The lesion was adjacent to a verrucous carcinoma
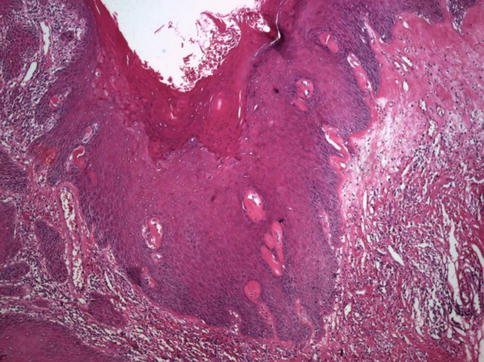
Fig. 5.8
This verrucous lesion was adjacent to a papillary carcinoma (left). Lichen sclerosus is also present (upper-right)
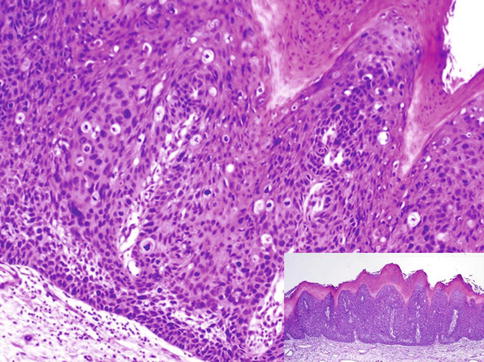
Fig. 5.9
Warthy-PeIN. Lesion at low power (Inset)
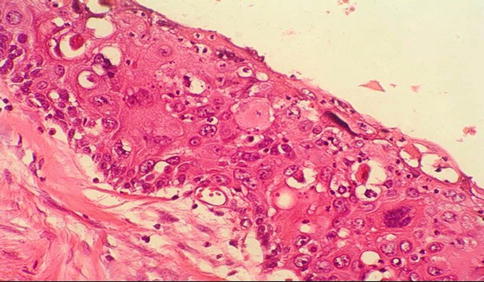
Fig. 5.10
High grade PeIN (Bowen-disease, carcinoma in situ)
Pathology
Differentiated or Simplex PeIN
Thickened acanthotic epithelium with elongated and anastomosing rete ridges. Subtle abnormal maturation (enlarged keratinocytes with abundant eosinophilic cytoplasm). Parakeratosis is frequent on the surface. Absence of koilocytosis.
Atypical basal cells with hyperchromatic nuclei. Occasionally, keratin pearl formation (in deep rete ridges). Prominent intercellular bridges (spongiosis and sometimes acantholysis).
Lichen sclerosus, chronic scarring, or other inflammatory dermatosis usually seen in adjacent epithelium.
Preferential association with HPV-unrelated variants of invasive squamous cell carcinoma.
Squamous hyperplasia or lichen simplex chronicus may enter differential diagnosis. Basilar atypia and abnormal maturation are not seen in reactive conditions.
Basaloid PeIN
Epithelium replaced by monotonous population of small immature cells with high nuclear/cytoplasmic ratio. Mitotic figures are numerous.
Warty PeIN
Epithelium replaced by pleomorphic cells with koilocytic changes including multinucleation, nuclei with irregular contours, perinuclear halo, and dyskeratosis. Undulating or spiky surface with atypical parakeratosis. Frequent mitoses.
Mixed PeIN (Warty/Basaloid)
Combined features of basaloid and warty types of PeIN. Warty/basaloid PeIN is usually seen adjacent to HPV-related variants of invasive squamous cell carcinoma (basaloid and warty)
Lower part of epithelium is replaced by small, basaloid cells, with high nuclear/cytoplasmic ratio. Upper portion shows features of warty PeIN.
Differential diagnosis includes condyloma acuminatum. Koilocytosis is restricted to upper epithelium, there is absence of nuclear pleomorphism, and mitoses are scant and confined to lower epithelium. Bowenoid papulosis may be indistinguishable from warty/basaloid PeIN on histology alone. Clinical correlation is essential.
5.2.2.2 Bowenoid Papulosis
HPV-related multifocal papular condition typically seen in anogenital region in young adults. May be pruritic, painful or asymptomatic. Usually transmitted via sexual contact. Related to high-risk HPV, especially HPV 16. Other HPV types have also been implicated.
Lesions are often small, brown, or red-colored and exhibit histologic spectrum of changes from dysplasia to in situ carcinoma. Usually affect skin of shaft and surface may be verruciform. May also affect epithelium of glans, coronal sulcus or foreskin. Spontaneous regression has been reported, but minority of lesions evolves to invasive squamous cell carcinoma.
Pathology
Proliferation of atypical basaloid cells that may be scattered throughout epidermis, often with preserved maturation. Koilocytic-like changes are also seen.
Atypia ranges from scattered atypical cells of low-grade dysplasia to full thickness atypia of the squamous epithelium. Most lesions are indistinguishable from squamous cell carcinoma in situ. Variable increased pigmentation of basal layer.
Differential diagnosis with squamous cell carcinoma in situ requires clinical correlation.
5.2.2.3 Squamous Hyperplasia
Most common epithelial change associated with penile cancer. Almost all keratinizing squamous cell carcinoma have associated squamous hyperplasia. There is thickening of mucosal squamous epithelium without cytologic atypia. It seems to be a reactive condition rather than specific entity. May be associated with reactive inflammatory conditions.
Clinically, it may be difficult to distinguish from PeIN. Micaceous balanitis and penile horn are clinically florid forms of squamous hyperplasia with prominent hyperkeratosis. Usually found in continuity or slightly distant from in situ or invasive carcinoma. Maybe precursor lesion of HPV-unrelated squamous cell carcinoma.
The differential diagnosis of squamous hyperplasia from differentiated PeIN or from extremely low-grade invasive neoplasms (e.g., pseudohyperplastic and verrucous carcinomas) may be particularly difficult.
Pathology
Whitish areas with irregular borders with acanthosis with orthokeratotic and minimal to absent parakeratosis with normal epithelial maturation. Chronic inflammation may be present. There is absence of cytologic atypia, koilocytosis, and intraepithelial keratin pearls. Frequently associated with differentiated PeIN and with lichen sclerosus in some cases. Usually found in association with usual, papillary, and verrucous squamous cell carcinoma (HPV-unrelated variants). Rarely present adjacent to condylomatous (warty) or basaloid squamous cell carcinoma.
Histological Subtypes
Flat is the most common subtype. There is non-atypical acanthosis, hyperkeratosis with orthokeratosis, and linear interface between basal layer and stroma.
Mixed form is the second most common type. There is presence of mixed areas of flat and papillary squamous hyperplasia.
Pseudoepitheliomatous hyperplasia is an uncommon pattern. There is acanthosis, downward elongated proliferation of rete ridges that appear detached from epithelium. Regular epithelial nests with peripheral palisading and stromal reaction are not prominent. Some cases are associated to papillary squamous hyperplasia.
Papillary, represents minority of cases with serrated appearance on low-power view and jagged interface with underlying stroma. There is non-atypical acanthosis with short hyperkeratotic papillae.
Verrucous pattern presents adjacent to verrucous carcinoma with marked acanthosis with no atypia, hyperkeratosis with hypergranulosis, and slight papillomatosis.
Differential diagnosis of squamous hyperplasia includes differentiated PeIN that shows aberrant keratinization with cytologic basal atypia.
Warty/basaloid PeIN with cytologic atypia with loss of squamous maturation and monotonous population of small cells with scant cytoplasm throughout epithelium in basaloid PeIN. Detection of HPV in high proportion of cases and immunohistochemical overexpression of p16 is the rule.
Pseudohyperplastic squamous cell carcinoma simulating pseudoepitheliomatous hyperplasia in which there is prominent stromal reaction and mass forming clinically.
Verruciform xanthoma, an exophytic lesion with acanthosis, hyperkeratosis, and “xanthoma” cells (foamy histocytes) in lamina propria.
Warty, papillary, and verrucous carcinomas show evidence of destructive stromal invasion. Diagnosis may be difficult in small biopsies (Figs. 5.11, 5.12, 5.13 and 5.14).
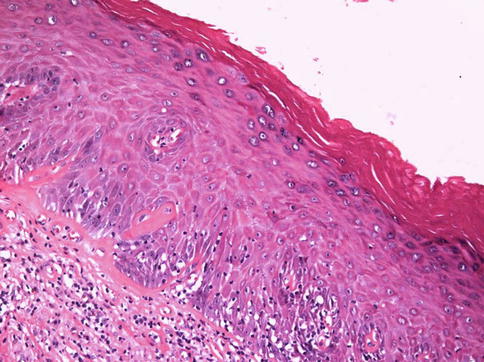
Fig. 5.11
Squamous hyperplasia
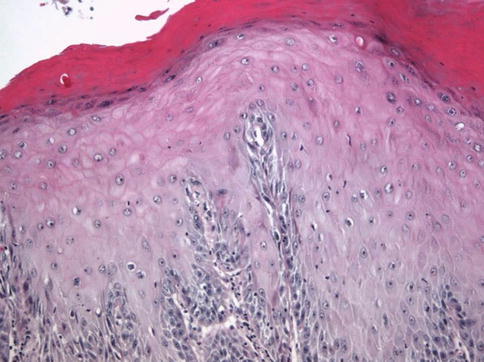
Fig. 5.12
Verrucous Squamous hyperplasia adjacent to verrucous carcinoma
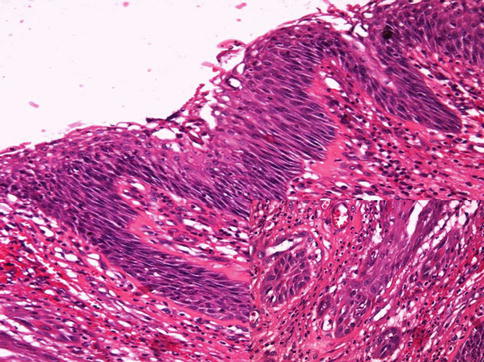
Fig. 5.13
Pseudoepitheliomatous hyperplasia with infiltrating nests (inset)
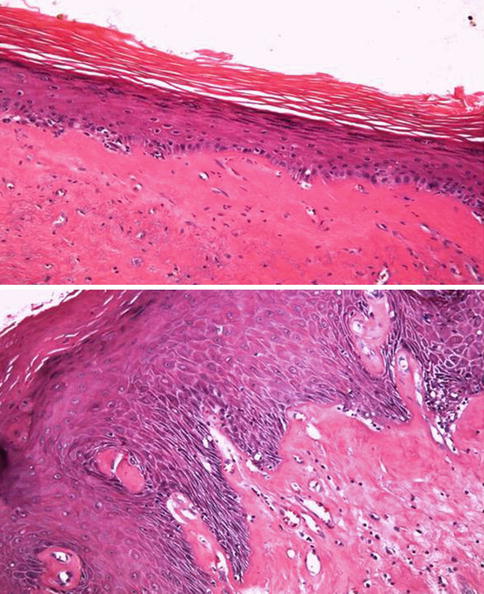
Fig. 5.14
Lichen sclerosus et atroficus (balanitis xerotica obliterans). Microscopic features (upper and lower view)
5.2.2.4 Lichen Sclerosus et Atrophicus (Balanitis Xerotica Obliterans)
Affect inner aspect of foreskin, glans, and perimeatal region, and may be focally hyperkeratotic. About 9 % of patients will develop penile squamous cell carcinoma, usual type.
Well-established lesions appear as white-gray, atrophic, and geographic with frequent erosion or ulceration. Early lesions appear as pink macules that become papules. It is a chronic process of unknown pathogenesis that tends to be broad and multifocal, and affect epithelium and superficial dermis/lamina propria.
Pathology
Typical case shows interface vacuolar or lichenoid dermatitis with thickened papillary dermis/lamina propria with band-like hyalinization (sclerosis). Scattered melanophages may be sen in superficial dermis and occasionally there is prominent edema of papillary dermis/lamina propria. Dermal-epidermal clefting or blisters may form secondary to marked basal cell vacuolar alteration.
In well-established lesions, a band-like lymphoid infiltrate underneath band of sclerosis. Early lesions show more superficial lymphoid infiltrate underneath epithelium without well-formed sclerotic band, but late lesions are sclerotic with minimal inflammation.
Rare cases of chronic lichen sclerosus may be associated with atypical foci in epithelium raising concern of epithelial dysplasia.
Lichen Planus enters differential diagnosis, shows a wedge-shaped hypergranulosis and band-like lymphoid infiltrate obscuring dermal-epidermal junction. Hyalinized band in superficial dermis/lamina propria is absent (Fig. 5.14).
5.2.3 Squamous Cell Carcinoma, Usual Type
Also known as epidermoid carcinoma, is and invasive carcinoma with keratinization and or intercellular bridges. Most common histologic subtype of penile squamous cell carcinoma (60 % of cases). HPV DNA is present in 25 % of cases.
Presents as solid mass most frequently affecting glans, with ulceration, pain, or bleeding. Superficial spreading is predominant pattern of growth. Size ranges 2–5 cm. Tumors exclusive of foreskin are rare (Table 5.6)
Table 5.6
Histologic types of squamous cell carcinoma of the penis
Squamous cell carcinoma, usual type
Variants of squamous cell carcinoma
Warty (condylomatous)
Basaloid
Verrucous
Pseudohyperplastic
Papillary
Sarcomatoid
Pseudoglandular (acantholytic)
Carcinoma cuniculatum
Adenosquamous
Mixed (hybrid)
Other
Pathology
Tumor nests of well to moderately differentiated squamous cell carcinoma with neoplastic cells with ample, eosinophilic, frequently keratinized cytoplasm and distinctive cellular borders.
Histologic grade heterogeneity is the rule and pure grade 1 or 3 tumors are very rare. Level of keratinization is the key for histologic grading. Only highest grade is considered for tumor grading. In some cases there are focal areas of spindle, trabecular, solid, or clear cells.
Differentiated PeIN, squamous hyperplasia or lichen sclerosus commonly found in adjacent areas. Associated basaloid and warty PeIN is rare.
Associated stromal reaction includes lymphocytes and plasma cells, and occasionally desmoplastic reaction. Vascular and perineural invasion is seen in about 30 % of cases, and extension to distal urethra in one-half of cases. Extension to multiple compartments is also common.
Pathologic prognostic factors include histologic grade, the level of infiltration (pathologic stage), vascular, lymphatic, and perineural invasion. Inguinal nodal metastases seen in about 30 % of patients. Some patients may experience tumor recurrences after definitive therapy.
Lesions entering differential diagnosis include pseudoepitheliomatous hyperplasia because of pseudoinfiltrative pattern of growth, but epithelial nests are orderly disposed, there is peripheral palisading and no stromal reaction and no or only mild cytologic atypia.
Pseudohyperplastic Carcinoma is extremely well differentiated, frequently multicentric, with inconspicuous peripheral palisading and prominent stromal reaction. Urothelial carcinoma of distal urethra is also an important differential; there is usually previous history of urothelial carcinoma, urothelial carcinoma in situ seen in adjacent areas. Immunohistochemistry with CK20, GATA3 and uroplakin-3 positive urothelial carcinoma and negative or only focal in squamous cell carcinoma may help in selected cases.
In mixed squamous cell carcinoma there are foci of usual squamous cell carcinoma intermingled with other subtypes. Verrucous carcinoma is the commonest (Figs. 5.15, 5.16, 5.17, 5.18, 5.19, 5.20, 5.21, 5.22, 5.23, 5.24 and 5.25)
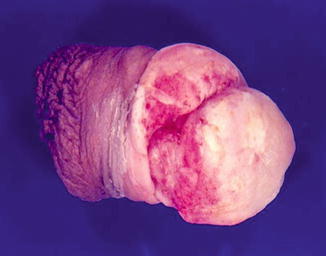
Fig. 5.15
Ulcerated squamous cell carcinoma with adjacent leukoplakia
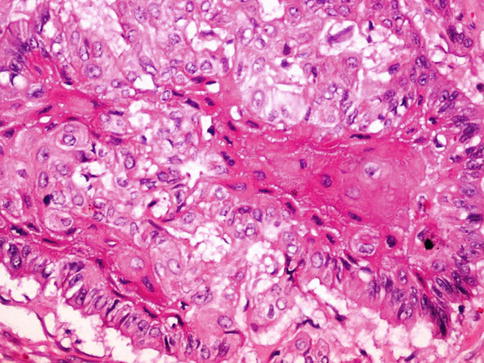
Fig. 5.16
Squamous cell carcinoma with intercellular bridges
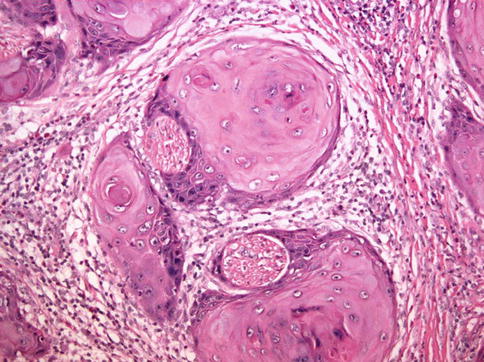
Fig. 5.17
Squamous cell carcinoma with perineural invasion
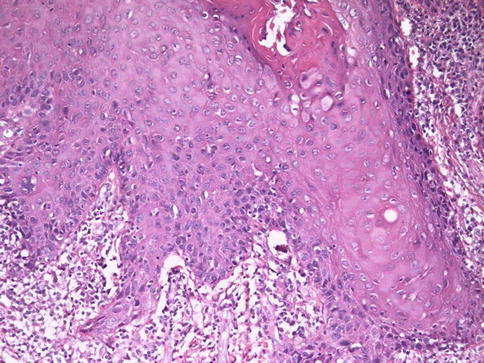
Fig. 5.18
Lack of maturation in squamous cell carcinoma
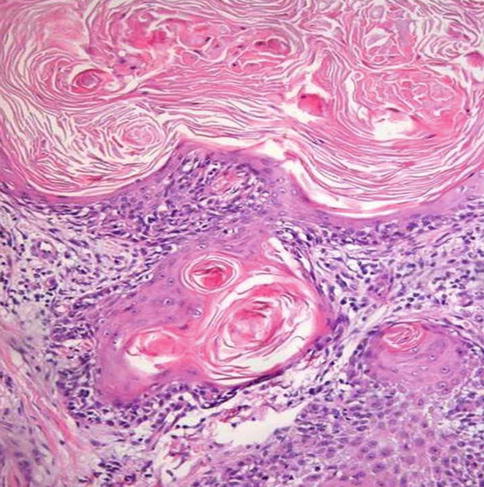
Fig. 5.19
Well differentiated-grade 1 squamous cell carcinoma
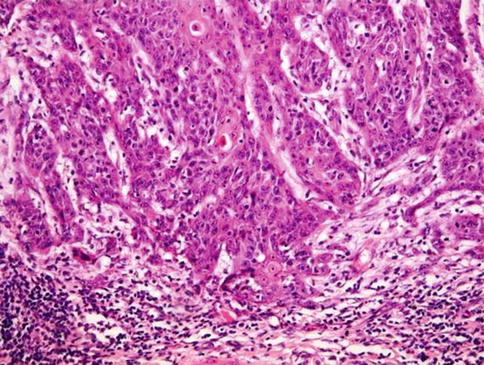
Fig. 5.20
Moderately differentiated-grade 2 squamous cell carcinoma
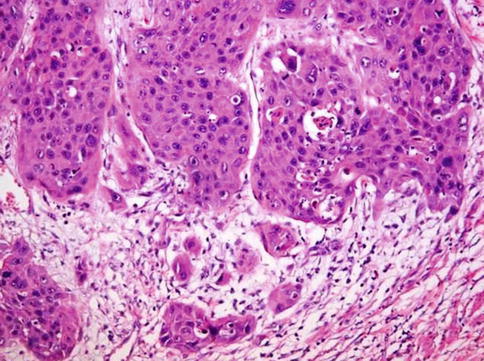
Fig. 5.21
Poorly differentiated grade 3 squamous cell carcinoma
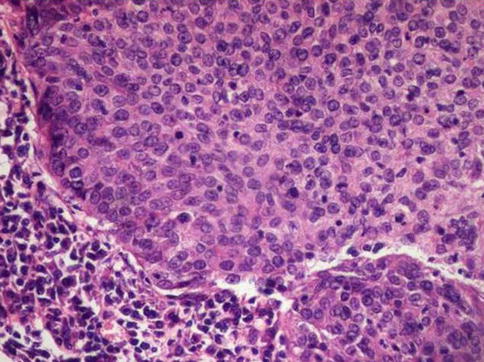
Fig. 5.22
Undifferentiated squamous cell carcinoma showing high mitotic ratio, and lack of keratin
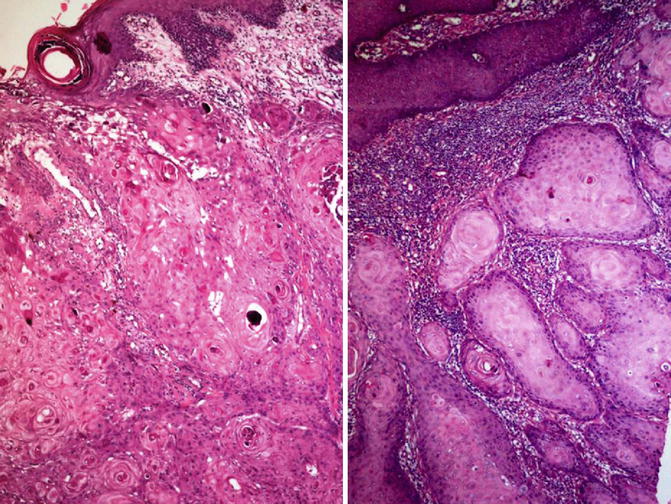
Fig. 5.23
Microscopic features of invasive squamous cell carcinoma with keratin (left) and invading nests (right)
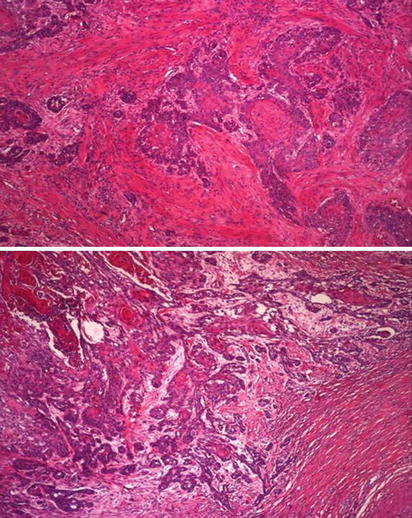
Fig. 5.24
Microscopic features of squamous cell carcinoma showing infiltrating neoplastic cords (upper) and associated stromal reaction (lower)
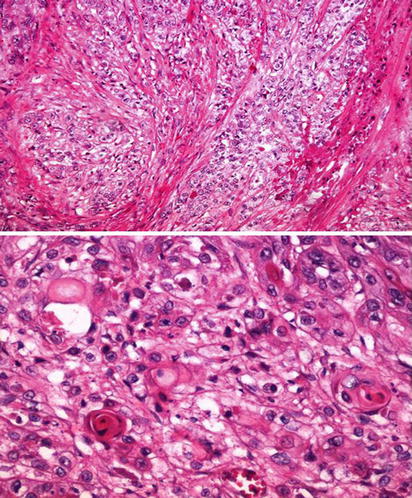
Fig. 5.25
Clear cell change in squamous cell carcinoma. Low (upper) and high (lower) power view
Metastatic squamous cell carcinoma to penis may occur. Most are from genitourinary origin. Prominent squamous differentiation may be seen in urothelial and less frequently in prostate cancer. Tumor nests located mainly in vascular spaces of penile erectile tissues but squamous mucosa covering glans, coronal sulcus, or inner foreskin is usually unaffected. Clinical correlation may be necessary.
5.2.4 Variants of Squamous Cell Carcinoma (Figs. 5.26, 5.27, 5.28, 5.29, 5.30, 5.31, 5.32, 5.33, 5.34, 5.35, 5.36, 5.37, 5.38, 5.39, 5.40, 5.41, 5.42, 5.43, 5.44, 5.45, 5.46, 5.47 and 5.48)
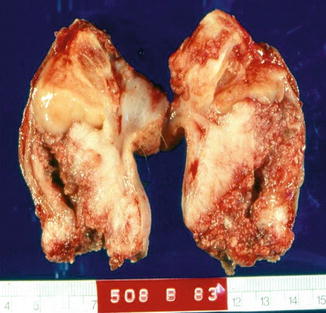
Fig. 5.26
Gross appearance of Warthy squamous cell carcinoma
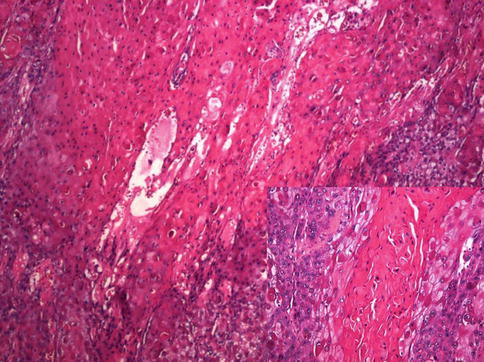
Fig. 5.27
Exophytic appearance of Warthy squamous cell carcinoma. High power view in inset
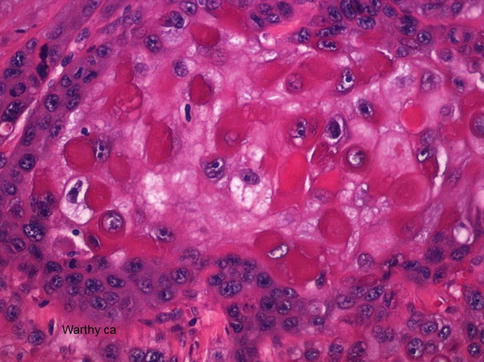
Fig. 5.28
Warthy squamous cell carcinoma with atypical parakeratosis and rhabdoid-like features
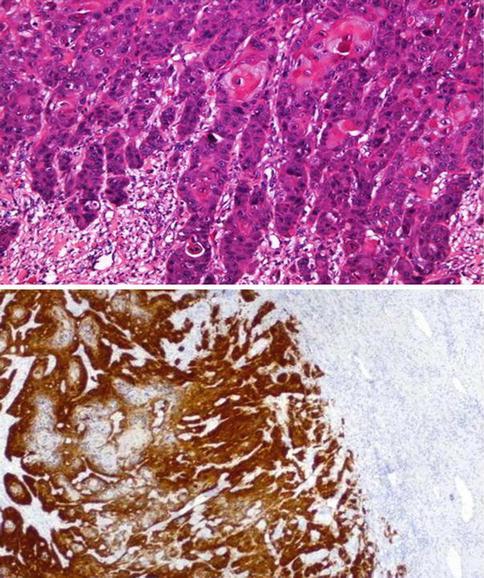
Fig. 5.29
Infiltrative aspect (upper) of Warty squamous cell carcinoma and p16 expression (lower)
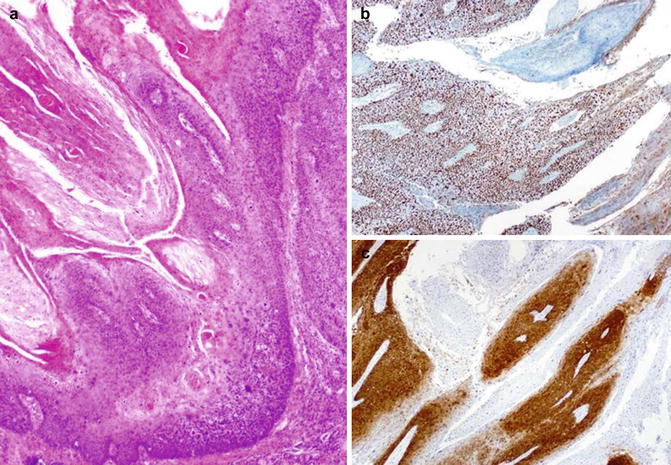
Fig. 5.30
Warthy squamous cell carcinoma (a) with ki67 proliferation (b) and p16 (c) expression
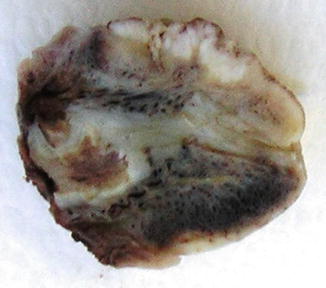
Fig. 5.31
Verrucous squamous cell carcinoma. Gross features
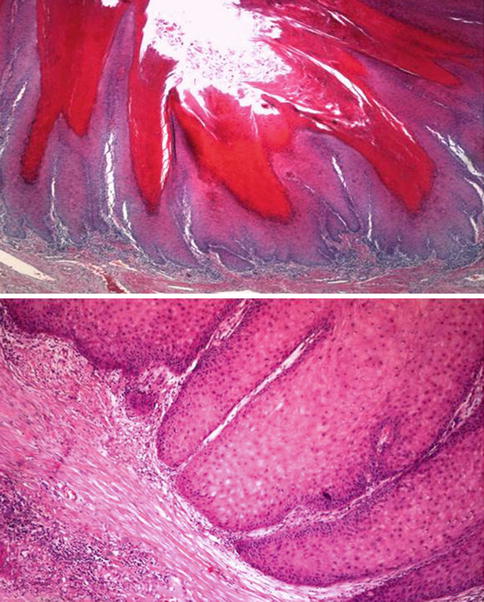
Fig. 5.32
Verrucous squamous cell carcinoma microscopic features (upper) and deep aspect of the lesion (lower)
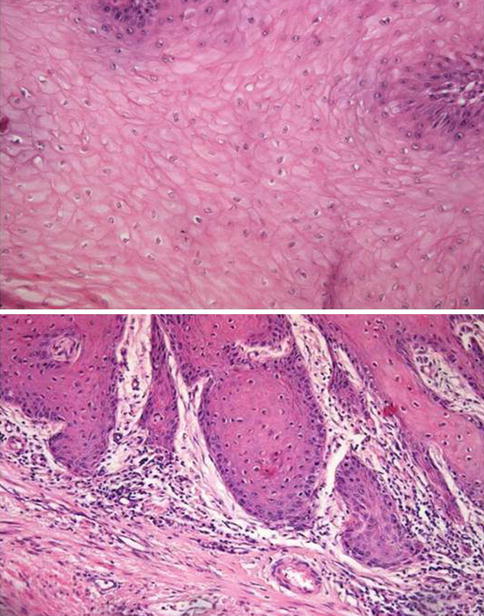
Fig. 5.33
Verrucous squamous cell carcinoma with focal koilocytic-like change
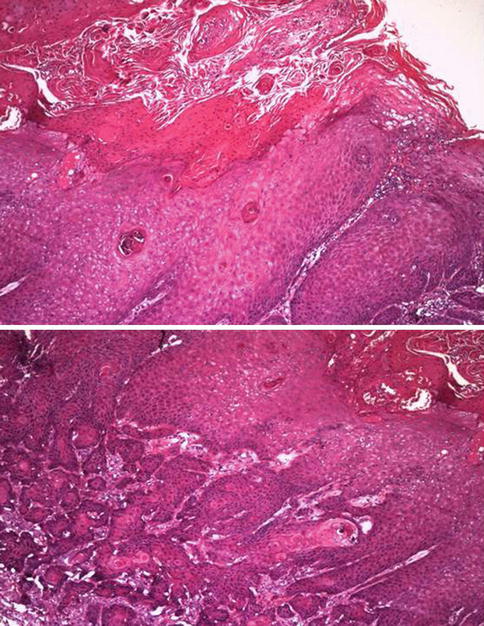
Fig. 5.34
Warthy squamous cell carcinoma simulating verrucous squamous cell carcinoma. Surface (upper) and infiltrative aspect (lower)
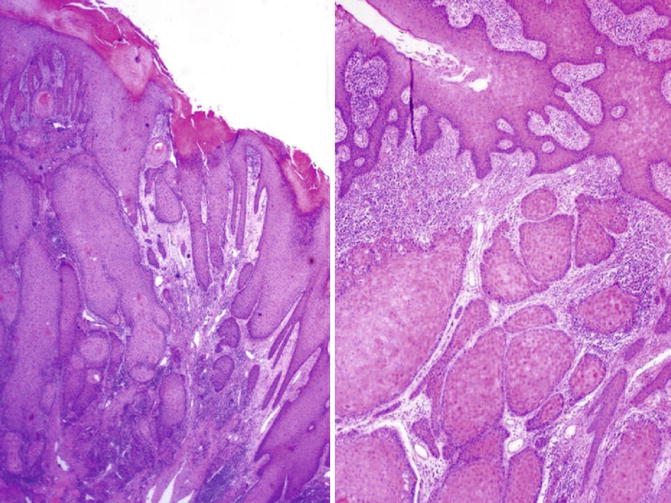
Fig. 5.35
Peudohyperplastic squamous cell carcinoma. Microscopic features (left) and infiltrating nests (right)
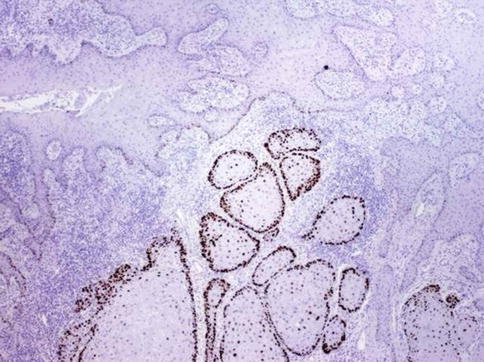
Fig. 5.36
p53 expression in peudohyperplastic squamous cell carcinoma
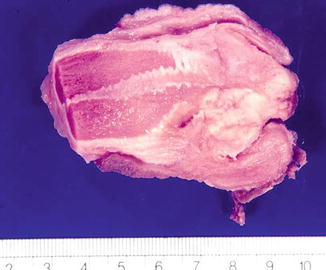
Fig. 5.37
Mixed-Basaloid squamous cell carcinoma. Gross features
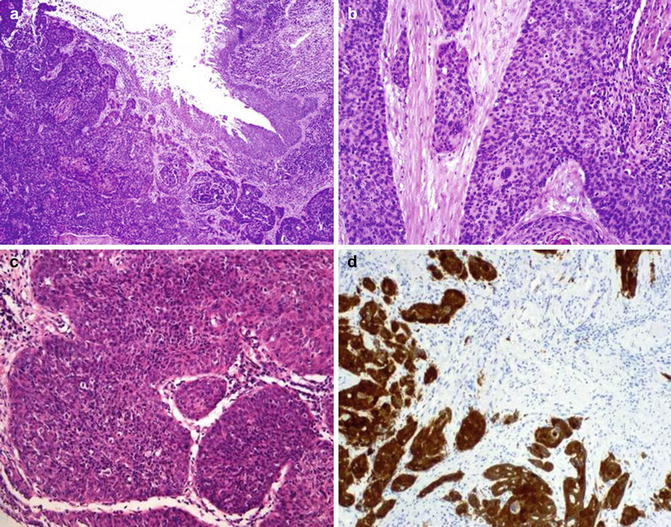
Fig. 5.38
Basaloid squamous cell carcinoma. Surface (a) infiltrative aspects (b, c), and p16 expression (d)
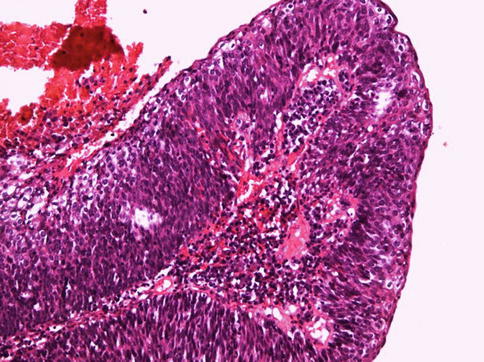
Fig. 5.39
Basaloid squamous cell carcinoma. Papillary architecture
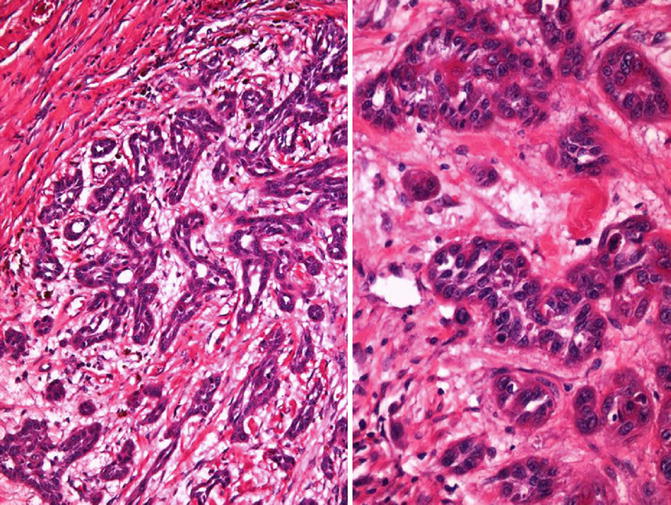
Fig. 5.40
Acantholytic/pseudoglandular squamous cell carcinoma. Low (left) and high (right) power
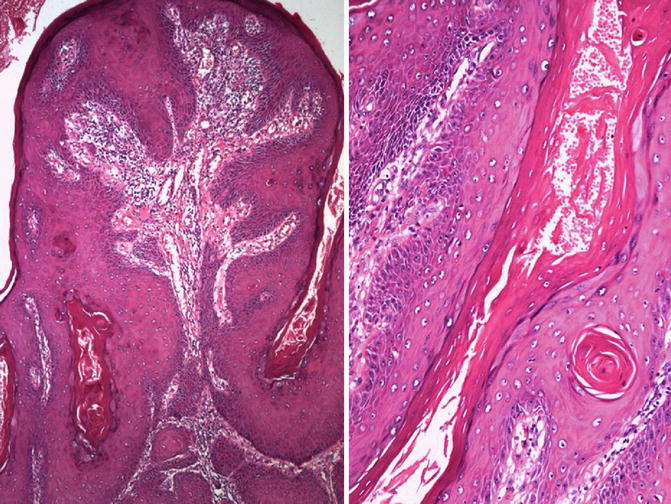
Fig. 5.41
Papillary squamous cell carcinoma at low (left) and high (right) power
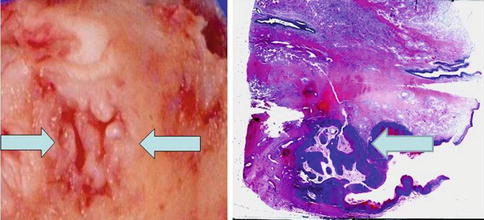
Fig. 5.42
Gross aspects of carcinoma Cuniculatum (left and right). Arrows point out gross (left) and microscopic view (right)
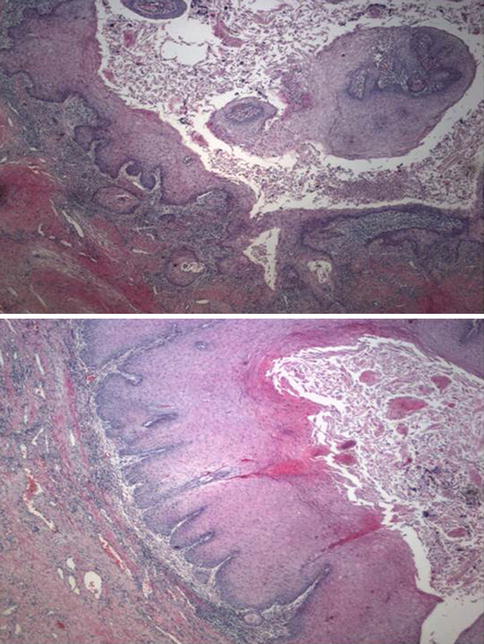
Fig. 5.43
Carcinoma Cuniculatum. Microscopic features (upper and lower)
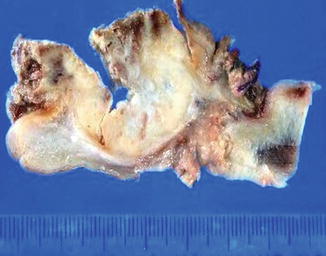
Fig. 5.44
Sarcomatoid squamous cell carcinoma. Gross features
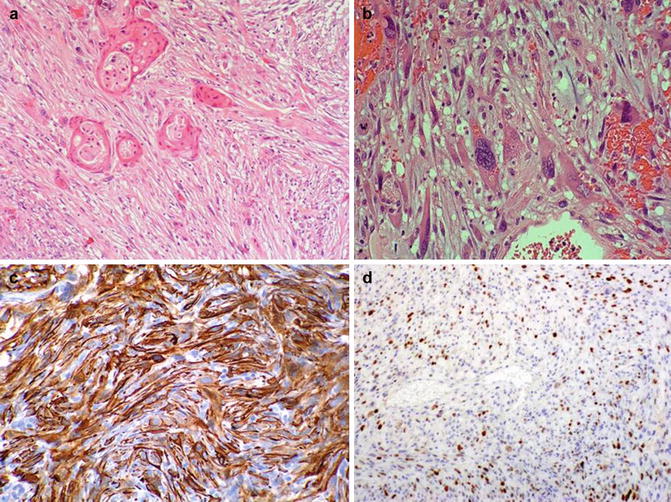
Fig. 5.45
Sarcomatoid squamous cell carcinoma. Microscopic features (a, b). Immunohistochemical expression of CKAE1/AE3 (c) and p63 (d)
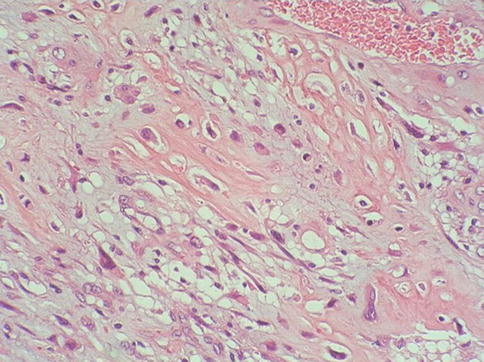
Fig. 5.46
Sarcomatoid squamous cell carcinoma with osteosarcomatous heterologous elements
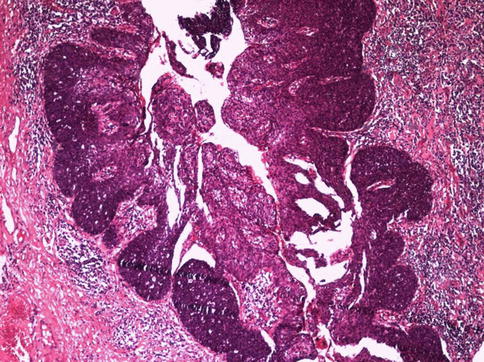
Fig. 5.47
Mixed-Basaloid squamous cell carcinoma
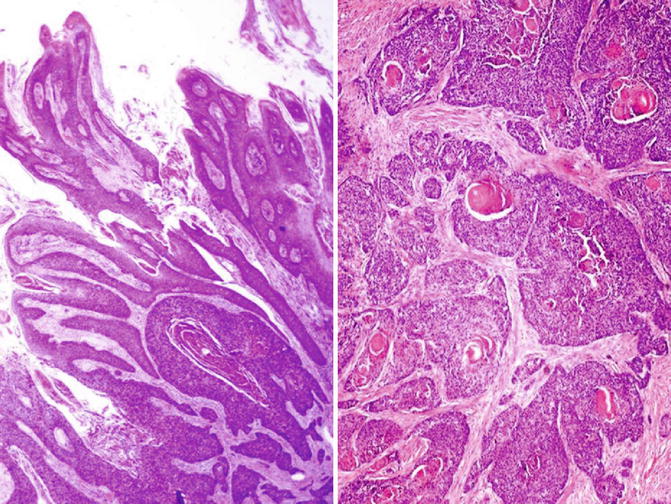
Fig. 5.48
Mixed Warty-Basaloid squamous cell carcinoma
5.2.4.1 Pseudohyperplastic Carcinoma
Rare nonverruciform low-grade squamous cell carcinoma preferentially affecting foreskin in elderly patients (eighth decade) strongly associated with lichen sclerosus et atrophicus. Most unrelated to HPV infection. It is often multicentric. Other concurrent tumor pattern may be verrucous carcinoma. Minority of cases may recur showing higher grade areas.
Pathology
Nonverruciform tumors usually affecting foreskin that may be hyperkeratotic flat or slightly elevated, often multicentric, on a background of lichen sclerosus. Mean size is 2 cm.
Extremely well-differentiated tumor that May be confined to subepithelial connective tissue or infiltrate dartos in foreskin. There is downward proliferation of keratinizing nests of squamous cells. Keratin-pearl formation within infiltrating nests. Occasional individual cell keratinization and mild atypia of basal cells. Lack of kolocytosis. Uneven or jagged infiltrative borders. Asymmetrical infiltrative nests surrounded by reactive fibrous stroma with mild inflammation. Adjacent epithelium may show differentiated PeIN.
Differential diagnosis may include pseudoepitheliomatous hyperplasia. Infiltration of dartos or corpus spongiosum is not seen in hyperplasia. Downward proliferation is more organized with elongated rete ridges. Keratin pearl formation is less frequent and inflammation is more prominent.
5.2.4.2 Warty (Condylomatous) Carcinoma
Slow-growing verruciform tumor usually affecting glans, but also, coronal sulcus, and/or foreskin. It is related to HPV infection (most frequent HPV16). Shares some gross and microscopic characteristics with condyloma but has definitive malignant potential. May represent up to 10 % of penis carcinomas and is more frequent in patients in their 50s.
It is placed as an intermediate aggressiveness between that of low risk verrucous or papillary carcinomas and high risk squamous cell carcinoma, usual type. It may present with inguinal nodal metastasis. Level of invasion and higher histologic grade are important prognostic factors.
Pathology
Rare verruciform, cauliflower-like tumor, with exo- to endophytic growth and papillomatous surface on cut sections. Usually affects at least two anatomical compartments (glans, coronal sulcus, and foreskin). Variable deep borders, pushing and/or jagged, usually penetrate into corpora spongiosum and or cavernosa.< div class='tao-gold-member'>Only gold members can continue reading. Log In or Register to continue
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree








