(1)
Division of Nephrology and Hypertension, Rutgers New Jersey Medical School, Newark, NJ, USA
Keywords
Immunology of transplantationImmunosuppressive drugsBlood group matchingPanel reactive antibodyCriteria for kidney allocationEstimated posttransplant survivalKidney donor profile indexInduction therapyFactors contributing to rejectionDesensitization proceduresGraft and patient survivalLymphoproliferative disordersSkin cancerVaccinationsInfectionsBK virusDelayed graft functionBanff classificationT cell-mediated rejectionAntibody-mediated rejectionRecurrent glomerular diseaseDe novo glomerular diseasePregnancy after transplantation1.
A 26-year-old man with ethylene glycol poisoning develops irreversible brain damage and acute kidney injury. He is on continuous venovenous hemodiafiltration for a week with no improvement in brain function. The family decides to donate his organs, and he is pronounced brain dead. Which one of the following immunologic events is expected to occur within the donor kidney at the time of donor harvest?
A.
Spillage of damage-activated molecular patterns (DAMPs) into the tissue milieu
B.
Activation of interstitial resident dendritic cells by DAMPs
C.
Brain death in the deceased donor causes swings in blood pressure (BP) with resultant ischemia/anoxia to the kidney
D.
Brain death is accompanied by massive release of cytokines that activate immune cells, endothelial and tubular epithelial cells of the kidney
E.
All of the above
The answer is E
The donor kidney consists of not only glomeruli, mesangium, tubular cells, and endothelium but also immune cells such as dendritic cells from the interstitium, which are phagocytic. These cells are usually dormant, and activated by low blood flow to the kidney following brain death. As a result of ischemia, cells in the donor kidney die and release such molecules as DAMPs. Examples of DAMPs are uric acid, ATP, heat-shock proteins, DNA, RNA, and glycosaminoglycans. DAMPs activate dendritic cells by binding to their receptors located on the epithelial, endothelial, and mesenchymal cells within the donor kidney. These receptors include toll-like receptors and nucleotide-binding oligomerization domain-like receptors. The interaction between DAMPS and their receptors triggers inflammatory cytokines which attract recipient inflammatory cells. Thus, the donor kidney is immunologically activated even before it is transplanted into the recipient.
One of the ways that resident dendritic cells become activated is through renal ischemia/anoxia caused by low blood flow to the donor kidneys. Brain death causes initially high BP followed by low BP. These swings in BP result finally into ischemia, which starts immunologic events in the donor kidney. Thus, E is correct.
Suggested Reading
Reis e Sousa C. Activation of dendritic cells: translating innate into adaptive immunity. Curr Opin Immunol 16:21–25, 2004.
John R, Nelson PJ. Dendritic cells in the kidney. J Am Soc Nephrol 18:2628–2635, 2007.
McKay DB, Park K, Perkins. What is transplant immunology and why are allografts rejected? In McKay DB, Steinberg SM (eds). Kidney Transplantation. A Guide to the Care of Kidney Transplant Recipients. New York, Springer, pp 25–39.
2.
In transplant immunobiology, the human major histocompatibility complex (MHC) plays an important role both in innate (natural) and adaptive immune systems. Regarding MHC and kidney transplantation, which one of the following statements is CORRECT?
A.
MHC comprises a set of genes that is located on the short arm of chromosome 6
B.
MHC genes are highly polymorphic (diverse), and each individual inherits one set of genes from each parent
C.
In humans, both MHC and major human leukocyte antigens (HLAs) are used interchangeably
D.
Among three classes of HLA genes, only class I and class II HLA genes are important for kidney transplantation
E.
All of the above
The answer is E
All of the statements are correct and also self-explanatory. Successful organ transplantation depends on the donor and recipient HLAs that are encoded by HLA (MHC) genes. These antigens are called alloantigens (a cell or tissue antigens that are present in some members of species but not in others), and are recognized as foreign antigens. The genes for these alloantigens (proteins) are clustered in MHC on the short arm of chromosome 6. Because MHC genes, also called HLA genes, are polymorphic, the products of these genes are also polymorphic. A new World Health Organization classification has been introduced in 2010 to include all HLA genes and their products.
HLA genes are classified into three classes: HLA class I, HLA class II, and HLA class III. HLA class I genes include HLA-A, HLA-B, and HLA-C genes and are present on cell surfaces of all nucleated cells. HLA class II genes include HLA-DP, HLA-DQ, and HLA-DR; the proteins of these genes are present in antigen-presenting cells such as dendritic cells, macrophages, B cells, endothelial cells, and some epithelial cells. Only HLA class I and HLA class II genes and their antigens participate in kidney transplants.
HLA class III genes encode complement proteins, heat-shock proteins, and tissue necrosis factors, and do not play a significant role in kidney transplantation.
In transplant immunobiology, the effector cells for rejection are T cells, although B cells are also important. The ability of T cells to recognize antigens is dependent on association of these antigens with either class I or class II HLA proteins. For examples, T helper (CD4) cells respond to antigens in association with class II proteins, whereas cytotoxic T (CD8) cells respond to class I proteins. Thus, either the rejection or acceptance of a transplant is dependent on either class I or class II HLA proteins on donor cells. Generally, class II proteins play a major role in these processes. Matching the class I and class II antigens between the donor and recipient is important for successful kidney transplantation. It should, however, be noted that many transplants are done successfully with six antigen mismatch as well.
Suggested Reading
Sayegh MH, Chandraker A. Transplantation immunobiology. In Taal MW, Chertow GM, Marsden PA, et al. (eds). Brenner & Rector’s The Kidney, 9th ed, Philadelphia, Elsevier Saunders, 2012, pp 2468–2494.
Fuggle SV, Taylor CJ. Histocompatibility in kidney transplantation. In Kidney Transplantation: Principles and Practice. Morris PJ, Knechtle SJ (eds), 7th ed, Edinburgh, Elsevier/Saunders, 2014, pp 142–160.
3.
Which one of the following immunologic barriers should be considered for any successful kidney transplantation?
A.
Blood group incompatibility
B.
Mismatched HLA (human leukocyte antigen) antigens
C.
Anti-donor HLA antibodies in the recipient
D.
Rhesus (Rh) factor positivity
E.
A, B, and C
The answer is E
Prior to kidney transplantation, both donor and recipient undergo certain tests to prevent rejection. These tests include blood type matching, HLA antigen matching, and anti-HLA antibody test. The first barrier to successful transplantation is blood group mismatch (A is correct). There are four types of blood groups: A, B, AB, and O. The most frequently found blood groups in the USA are A and O (42–44 %). The A and B groups are glycosylated, whereas group O lacks glycosylation. Individuals with A or B blood types produce natural antibodies to the opposite type, and individuals with group O produce antibodies to both A and B. Because group O lacks glycosylated moiety, both A and B fail to produce antibodies to group O. Thus, group O grafts can be transplanted into patients with blood types A, B, and AB. However, it is not a common practice for ethical reasons in order to prevent depletion of available transplants for type O recipients, who must receive an O transplant. When ABO incompatible kidney is transplanted, it is immediately rejected because of preformed anti-A and/or anti-B antibodies. The following table (Table 11.1 ) shows the blood compatibility between donor and recipient blood groups.
Table 11.1
Blood type compatibility
Recipient blood type | Donor blood type compatible with recipient |
|---|---|
A | A, O |
B | B, O |
AB | A, B, AB, O |
O | O (A*) |
The second barrier is mismatched HLA antigens. Despite many advances, HLA matching continues to be a major determinant of graft survival. The survival of grafts with no mismatches is much greater than those with one mismatch. However, concerns were raised about the HLA antigen mismatch, as no benefit in survival of grafts was observed with HLA matching in African Americans. A recent review concludes that HLA matching should continue because it allows the use of lower dosages of immunosuppressive agents. This conclusion was based on: (1) HLA matching overrides the impact of cold ischemia time (as longer time has negative impact on graft survival) on graft survival; (2) HLA mismatched patients were frequently hospitalized for malignancies due to high dosages of immunosuppressive agents; and, finally, HLA mismatches at Class II level (HLA-DR) appear to be associated with the development of de no development of donor-specific antibody 4.6 years after transplantation. Thus, HLA matching is important (B is correct).
The third barrier is the presence of anti-donor HLA antibodies. If not desensitized, these donor-specific antibodies cause hyperacute rejection and also limit the survival of the allograft. Prior to transplantation, crossmatch testing is done for the presence or absence of these antibodies (C is correct).
The final barrier is the presence of minor HLAs, which are not well studied. The requirement of reduced immunosuppression in recipients of HLA identical siblings suggests the presence of such minor HLAs.
The rhesus (Rh) factor either negative or positive is not an issue in kidney transplantation, as the kidney does not express this blood group. Thus, D is incorrect
Suggested Reading
Süsal C, Opelz G. Current role of human leukocyte antigen matching in kidney transplantation. Curr Opin Transplant 18:438–444, 2013.
Santos RD, Langewisch ED, Norman DJ. Immunological assessment of the transplant patient. In Weir MR, Lerma EV (eds). Kidney Transplantation. Practical Guide to Management. New York, Springer, 2014, pp 23–34.
4.
In addition to the above tests, crossmatch testing is necessary prior to surgical transplantation to detect anti-HLA antibodies in the recipient. Which one of the following tests is NOT used to detect anti-HLA antibodies in the recipient?
A.
Complement-dependent cytotoxicity (NIH-CDC) test
B.
Antihuman globulin (AHG-CDC) enhanced test
C.
T and B cell flow cytometric test
D.
Solid phase bead or ELISA assay
E.
Panel Reactive Antibody (PRA) test
The answer is E
Crossmatching test was introduced in transplant technology to identify the antibodies in the serum of recipients that can cause hyperacute rejection or early graft failure. The oldest test is the NIH-CDC with very low sensitivity; however, the sensitivity of this test has subsequently been increased by the addition of AHG. Further sensitivity is increased by flow cytometric crossmatch testing, and the highest sensitive test is the solid phase bead or ELISA assay (A–D are correct). The PRA test is a screening test that is used for patients who are on the nationwide deceased donor list. Thus, E is incorrect.
The following table (Table 11.2 ) gives a list of various assays for detection of alloantibodies in the recipient.
Table 11.2
Various alloantibody detection assays
Assays | Sensitivity |
|---|---|
Screening tests | |
Panel reactive antibody (T cell only) | |
Donor-specific alloantibody detection | |
Solid phase beads or ELISA | |
Class I HLA antibody tests | |
T cell cytotoxicity (NIH-CDC) | Very low |
T cell AHG-CDC | Low |
T cell flow cytometric test | High |
Solid phase bead or ELISA | Very high (highest) |
Both class I and class II HLA antibodies tests | |
B cell cytotoxicity (NIH-CDC) | Low |
B cell flow cytometric test | High |
Solid phase bead or ELISA | Very high (highest) |
The reader is referred to the following suggested reading for technical aspects of these tests.
Suggested Reading
Mulley WR, Kanellis J. Understanding crossmatch testing in organ transplantation: A case-based guide for the general nephrologist. Nephrology 16:125–133, 2011.
Fuggle SV, Taylor CJ. Histocompatibility in kidney transplantation. In Kidney Transplantation: Principles and Practice. Morris PJ, Knechtle SJ (eds), 7th ed, Edinburgh, Elsevier/Saunders, 2014, pp 142–160.
Santos RD, Langewisch ED, Norman DJ. Immunological assessment of the transplant patient. In Weir MR, Lerma EV (eds). Kidney Transplantation. Practical Guide to Management. New York, Springer, 2014, pp 23–34.
5.
Patients awaiting cadaveric kidneys have their serum screened for antibodies. Which one of the following antibody testing is MOST important in screening of these patients?
A.
Blood group B antibody
B.
Blood group A antibody
C.
Blood group AB antibody
D.
Panel reactive antibody (PRA)
E.
None of the above
The answer is D
The most important screening test in the recipient awaiting nationwide deceased donor kidney is the PRA test, which is expressed from 0 to 100 % (D is correct). This test is done by testing the recipient serum with lymphocytes from individuals who are considered representatives of the general population. Most laboratories perform the test by using the lymphocytes from 30 to 50 subjects. A 0 % of PRA indicates no antibodies against the general population. On the other hand, if the recipient has a PRA of 30, it tells that 30 % of the population is not a suitable donor of the kidney. The higher the percentage, the longer is the waiting time and less likely to have a kidney transplant. It is suggested that this test should be discouraged because of falsely elevated percentage points. Also, this screening test should not be confused with crossmatch testing. Tests from A to C are not screening tests
Suggested Reading
Murphey CL, Forsthuber TG. Trends in HLA antibody screening and identification and their role in transplantation. Expert Rev Clin Immunol 4:391–399, 2008.
Milfrod EL, Guleria I. What is histocompatibility testing and how is it done? In McKay DB, Steinberg SM (eds). Kidney Transplantation. A Guide to the Care of Kidney Transplant Recipients. New York, Springer, pp 41–55.
6.
The new deceased donor kidney allocation system has been revised and implemented on December 4, 2014. Which one of the following variables is included in designing Estimated Posttransplant Survival (EPTS) ?
A.
Age of the patient
B.
Time on dialysis
C.
Diabetes status
D.
Prior organ transplant
E.
All of the above
The answer is E
The revised system of kidney allocation has been developed with the participation of transplant professionals and people who have personal experience with donation and transplantation. This system was built on two designs to improve the allocation of the kidney: EPTS and KDPI (Kidney Donor Profile Index ). EPTS is a tool to estimate the anticipated posttransplant survival in the recipient. It is built on four variables: age of the patient, time on dialysis in years, diabetes status, and the number of prior solid organ transplants (E is correct). A score is calculated based on these variables, which is expressed as percentage from 0 to 100 %. The transplanted kidney is expected to last longer in patient with a score <20 % compared to a patient with a score >20 %.
KDPI incorporates ten donor factors which provide the relative risk of posttransplant kidney graft failure. Thus, KDPI measures donor quality. KDPI is applicable to only deceased donors and not living donors. Lower KDPI scores indicate high donor quality, whereas higher KDPI scores are associated with lower donor quality. Table 11.3 shows the ten donor factors (variables).
Table 11.3
Donor factors for KDPI
Age |
Height |
Weight |
Ethnicity |
History of hypertension |
History of diabetes |
Cause of death |
Serum creatinine |
Hepatitis C virus status |
Donation after circulatory death status (brain or cardiovascular death) |
EPTS scores are used to divide patients on the waiting list into the top 20 % and remaining 80 %. The top 20 % will receive the kidney with the longest estimated posttransplant survival . Based on EPTS and KDPI, the kidneys are allocated in four sequences:
Sequence A: Top 20 % KDPI kidneys to 20 % EPTS candidates
Sequence B: Kidneys with KDPI between 20 and 35 %, pediatric candidate
Sequence C: Kidneys with KDPI from 20 to 85 %
Sequence D: Kidneys with KDPI >85 %, revamped expanded criteria donor (ECD). (older candidates benefit from sequence D)
Thus, this new allocation kidney system may fulfil the principles of utility and equity (justice).
Suggested Reading
Rao PS, Schaubel DE, Guidinger MK, et al. A comprehensive risk quantification score for deceased donor kidneys: The kidney donor risk index. Transplantation 88:231–236, 2009.
Friedenwald JJ, Samana CJ, Kasiske BL, et al. The kidney allocation system. Surg Clin N Am 93:1395–1406, 2013.
7.
Which one of the following statements regarding the presence of antibodies and rejection of transplanted kidney is CORRECT?
A.
IgG antibodies against HLA-A and B antigens result in acute rejection
B.
IgM antibodies against HLA-A and B antigens in the current rather than past serum are highly predictive of rejection
C.
Autoimmune disease (such as lupus)-induced antibodies do not play an important role in rejection
D.
Medication (hydralazine, quinidine)-induced antibodies have significant role in rejection
E.
A, B, and C
The answer is E
All of the above statements except D are correct (E). Lupus or medication-induced antibodies are of IgM isotype, and are not important in kidney transplantation as they do not mediate rejection (D is incorrect). However, IgM antibodies can cause a positive crossmatch.
Suggested Reading
McKay DB, Park K, Perkins. What is transplant immunology and why are allografts rejected? In McKay DB, Steinberg SM (eds). Kidney Transplantation. A Guide to the Care of Kidney Transplant Recipients. New York, Springer, pp 25–39.
Milfrod EL, Guleria I. What is histocompatibility testing and how is it done? In McKay DB, Steinberg SM (eds). Kidney Transplantation. A Guide to the Care of Kidney Transplant Recipients. New York, Springer, pp 41–55.
8.
A 42-year-old Caucasian woman donates her kidney to her son with Alport syndrome. At the time of donation, her eGFR was 96 mL/min, BP 128/74 mmHg, and normal glucose and urinalysis. Currently, she is being followed by a nephrologist for health-related issues. Which one of the following statements regarding posttransplant medical conditions is CORRECT?
A.
Her eGFR may decrease but the decrease is comparable to that of controls (with two kidneys)
B.
She may develop albuminuria/proteinuria (proteinuria >500 mg/day) which is higher than controls
C.
She is likely to develop hypertension (blood pressure >140/90 mmHg)
D.
Her progression to ESRD is difficult to assess, as the development of ESRD is controversial
E.
All of the above
The answer is E
Posttransplant development of complications is rather common. The kidney function seems to be preserved, and the expected decline in eGFR is similar to those of controls (A is correct). However, the risk of having a GFR <60 mL/min was associated with older age, females, and higher BMI. Studies have shown that follow-up of live donors for albuminuria/proteinuria showed that approximately 12 % develop albuminuria/proteinuria after 7–12 years (B is correct). Development of hypertension is common, and reported to occur in approximately 30 % of donors (C is correct). The results of progression to ESRD after donation are mixed. Some studies have shown no progression, and some studies reported progression. In the latter studies, the incidence of ESRD after 15 years of donation was found to be 0.3 %, as compared with 0.04 % in controls (a 15-fold increase). Also, another study showed a 7.4-fold increase in the development of ESRD after 7.8 years post-donation, as compared with controls. Thus, the progression to ESRD is controversial (D is correct).
Suggested Reading
Ibrahim HN, Foley R, Tan L, et al. Long-term consequences of kidney donation. N Engl J Med 360:459–469, 2009.
Reule S, Matas A, Ibrahim HN. Live donor transplantation. In Weir MR, Lerma EV (eds). Kidney Transplantation. Practical Guide to Management. New York, Springer, 2014, pp 75–84.
9.
A 40-year-old woman is willing to donate her kidney to her daughter with type 2 diabetes. Her BMI is 38 kg/m2, BP 142/92 mmHg, and 24-h proteinuria of 310 mg. She has a history of recurrent calcium oxalate stones. Which one of the following statements regarding her kidney donation is CORRECT?
A.
Her excess weight excludes her from donation
B.
Her uncontrolled BP may exclude her from donation
C.
Her proteinuria indicates underlying glomerular disease
D.
She is not at risk for surgical complications compared to lean donors
E.
All of the above
The answer is E
All of the above statements are correct (E). A BMI >35 kg/m 2 is considered a relative contraindication for donation at many transplant centers. The obese individuals are advised to lose weight to <25 kg/m 2 before donation, as obesity >25 kg/m 2 is associated with ESRD. Uncontrolled hypertension (ambulatory BP >140/90 mmHg) is also a relative contraindication for donation. However, the kidney donation is accepted provided the donors are >50 years of age, eGFR ≥80 mL/min, and normal urinalysis.
Proteinuria of 310 mg/day generally indicates an underlying glomerular disease. Since proteinuria is a risk factor for progression of kidney and cardiovascular disease, donor kidney is not accepted from those with proteinuria >300 mg/d with or without hypertension. Generally, the obese subjects experience more surgical complications during nephrectomy than lean subjects; however, some studies reported no surgical risk at all. Recurrence of kidney stones is a contraindication for kidney donation. Weight loss may improve many of the conditions of metabolic syndrome and make the obese subjects suitable for kidney donation. The donor has many health-related issues that make her unsuitable for kidney donation.
Suggested Reading
Srinivas TR, Meier-Kriesche H-U. Obesity and kidney transplantation. Sem Nephrol 33:34–43, 2013.
Reule S, Matas A, Ibrahim HN. Live donor transplantation. In Weir MR, Lerma EV (eds). Kidney Transplantation. Practical Guide to Management. New York, Springer, 2014, pp 75–84.
10.
A 50-year-old woman, mother of three children, develops ESRD and on hemodialysis for 2 years. Prior to transplantation, she was found to have higher titers of anti-HLA antibodies on crossmatching. Which one of the following exposures is associated with the development of antibodies is CORRECT?
A.
Pregnancy
B.
Blood transfusion
C.
Prior transplantation
D.
Autoimmune disease
E.
A, B, and C
The answer is E
Unlike anti-ABO antibodies, anti-HLA antibodies do not occur naturally. The exposure of HLA antigens and subsequent development of anti-HLA antibodies are seen in three conditions: pregnancy, blood transfusion, and prior transplantation (E is correct). Autoimmune-induced antibodies (IgM isotype) do not participate in kidney transplantation. Thus, D is incorrect.
Suggested Reading
Santos RD, Langewisch ED, Norman DJ. Immunological assessment of the transplant patient. In Weir MR, Lerma EV (eds). Kidney Transplantation. Practical Guide to Management. New York, Springer, 2014, pp 23–34.
11.
Because of the presence of anti-HLA antibodies, the above recipient is highly sensitized. Which one of the following interventions is helpful in desensitizing (removal of antibodies) the patient?
A.
Plasmapheresis
B.
Intravenous IG
C.
Immunoadsorption
D.
Rituximab
E.
All of the above
The answer is E
All of the above intervention modalities have been tried alone or in combination to desensitize the patient, and mixed results were obtained (E is correct). However, patients with very high titers still retain some antibodies despite the use of these techniques. Acute and chronic rejections of allografts are expected in patients with elevated titers of antibodies. However, some patients maintain graft survival and renal function for several years after transplantation despite the presence of some anti-HLA antibodies. Splenectomy, eculizumab (inhibitor of C5a and C5b that prevents the formation of the terminal complement complex C5b-9), and proteosome inhibitor (bortezomib) have also been used as desensitization procedures.
Suggested Reading
Jordan SC, Pescovitz MD. Presensitization: the problem and its management. Clin J Am Soc Nephrol 1:421–432, 2006.
Marfo K, Lu A, Ling M, et al. Desensitization protocols and their outcome. Clin J Am Soc Nephrol 6:922–936, 2011.
Abu Jawdeh BG, Cuffy MC, Alloway RR, et al. Desensitization in kidney transplantation: review and future perspectives. Clin Transplant 28:494–507, 2014.
12.
Which one of the following effects is expected to occur after desensitization?
A.
Increase in fungal infections
B.
Increase in acute antibody-mediated rejection (AMR)
C.
Increase in chronic allograft nephropathy
D.
Increase in patient survival
E.
All of the above
The answer is E
Desensitization procedures are expensive, and these procedures have been associated with increased fungal infections, increased rate of AMR, and higher subclinical and chronic antibody-mediated rejection compared to nonsensitized or placebo groups. A study from Johns Hopkins reported increased patient survival following plasmapheresis/IVIG treatment , as compared with those patients on dialysis. Thus, E is correct.
Suggested Reading
Montgomery RA, Lonze BE, King KE, et al. Desensitization in HLA-incompatible kidney recipients and survival. N Engl J Med 365:318–326, 2011.
Marfo K, Lu A, Ling M, et al. Desensitization protocols and their outcome. Clin J Am Soc Nephrol 6:922–936, 2011.
13.
A 72-year-old nonobese woman wants to donate her kidney to her only daughter of 50-years-old with hypertensive nephrosclerosis and eGFR <15 mL/min. She approached several transplant centers, and one center decides to perform preemptive transplantation. Which one of the following statements regarding graft and recipient survival from older donors is CORRECT?
A.
Graft survival is similar between older and younger donors
B.
Mortality rate is not higher in kidney recipients from older donors compared to younger donors
C.
Kidneys from older donors (>70 years) should not be accepted for transplantation
D.
Transplantation of kidneys from donors >70 years of age is associated with less graft survival and increased mortality
E.
Crossmatch is not necessary when transplantation is done between mother and daughter
The answer is D
Several studies have shown that kidney donation from older individuals had increased rejection rate, lower graft function, and poor graft survival when compared to younger donors (A is false). However, other studies have shown no difference in graft survival between older and younger donors. As far as recipient death is concerned, donor age is a risk factor for recipient death, which is attributed to age-related decrease in renal function (or increase in serum creatinine) and associated cardiovascular disease (B is false). Recently, some centers accept kidney donation from subjects >70 years of age (C is false). Crossmatch is mandatory even between living-related individuals to evaluate donor-specific antibodies (E is false).
A study by Berger et al. showed that the graft survival from live donors aged 70 years or older was much lower than the graft survival from donors aged 50–59 years after 1, 5, and 10 years of transplantation. Similarly, patient survival was much lower in those who received kidneys from older (>70 years) compared to the recipient survival who received kidneys from younger group (50–59 years). The actual data are shown below (Table 11.4 ):
Table 11.4
Renal data
Years | Graft survival (%) older/younger | Recipient survival (%) |
|---|---|---|
1 | 7.4/5.0 | 93.1/96.4 |
5 | 14.9/12.0 | 74.5/83.3 |
10 | 38.3/21.6 | 56.2/64.2 |
Thus, graft failure was higher and patient survival was lower in recipients who received kidneys from donors aged >70 years compared to those who received kidneys from younger donors (D is correct). Interestingly, there was no difference in survival between donors compared to the survival of age-matched general population.
Suggested Reading
Berger JC, Muzaale AD, James N, et al. Living kidney donors ages 70 and older: Recipient and donor outcomes. Clin J Am Soc Nephrol 6:2887–2893, 2011.
Reule S, Matas A, Ibrahim HN. Live donor transplantation. In Weir MR, Lerma EV (eds). Kidney Transplantation. Practical Guide to Management. New York, Springer, 2014, pp 75–84.
14.
A 40-year-old woman is evaluated for kidney donation. All the pertinent tests are negative except for microscopic hematuria. She had menstrual cycle 15 days ago. Urine sediment shows six dysmorphic RBCs on phase contrast microscopy. Which one of the following tests you recommend for this donor?
A.
Ultrasound of the kidneys
B.
CT of the kidneys
C.
Renal biopsy
D.
Malignancy work-up
E.
Work-up for renal stones
The answer is C
There are no prospective studies that evaluated the issue of hematuria in live donors, although case reports suggest that donor kidneys with glomerular abnormalities had good short-term outcomes both in the donor and recipients.
In general, isolated hematuria with three to five isomorphic RBCs/HPF is not a contraindication for kidney donation. On the other hand, dysmorphic RBCs indicate glomerular origin and disease. In donors with dysmorphic RBCs, a renal biopsy is indicated for evaluation of IgA nephropathy, thin basement membrane, or Alport syndrome (C is correct). If the donor has IgA nephropathy, she is prohibited from donation. Some centers may accept kidney donation from patients with thin basement membrane nephropathy if the donor does not have proteinuria, hypertension, or reduced renal function. If the donor is a carrier for Alport syndrome and the renal function is normal without proteinuria, or hypertension, she may donate the kidney even in the presence of microscopic hematuria. On the other hand, if the biopsy shows pathology of Alport syndrome, the donor should not be accepted because the recipient may develop anti-GBM-antibody disease. All the other tests in this donor may not provide adequate information.
Suggested Reading
Yachnin T, Iaina A, Schwartz D, et al. The mother of an Alport’s syndrome: a safe kidney donor? Nephrol Dial Transplant 17:683, 2002.
Ierino F, Kanellis J. Donors at risk: haematuria. Nephrology 15: S111–S113, 2010.
15.
The following diagram shows the activation of the recipient adaptive immune system following transplantation. Immunosuppressive drugs act on several steps of this activated adaptive immune system. Match the letters shown in the figure with the appropriate immunosuppressive agents (Fig. 11.1 ):
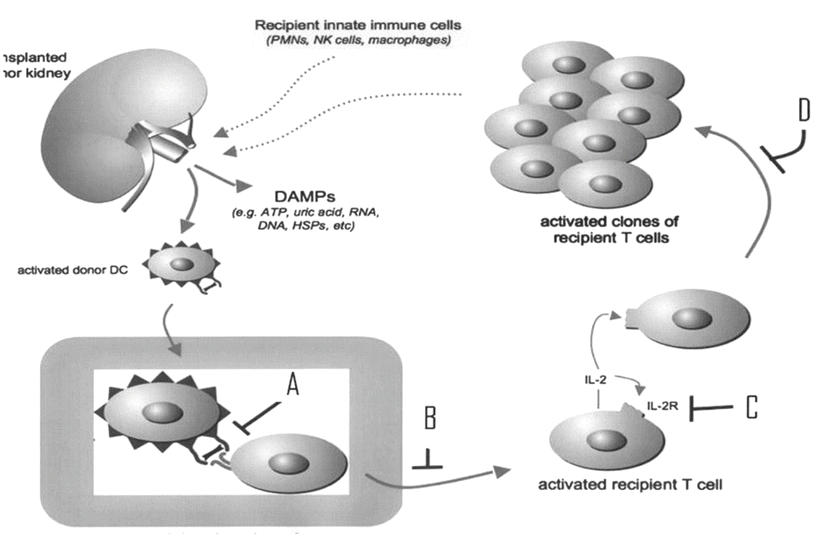

Fig. 11.1
Activation of the recipient adaptive immune system following donor kidney transplantation (adapted from McKay DB, Steinberg SM, with permission from Springer)
Letter | Immunosuppressive agents |
|---|---|
A | 1. OKT3, ATG, belatacept, alefacept |
B | 2. Cyclosporine, tacrolimus |
C | 3. Daclizumab, basiliximab |
D | 4. Mycophenolate mofetil, azathioprine, sirolimus, JAK3 inhibitors |
Answers: A = 1; B = 2; C = 3; D = 4
As soon as the donor kidney is transplanted, several important reactions occur, as discussed in question 1. First, the recipient is exposed to DAMPs (damage-activated molecular patterns) released from the donor kidney and activates dendritic cells. The activated dendritic cells enter the lymph nodes of the recipient where they encounter naïve T cells and activate them. This stimulation also requires simultaneous costimulatory molecules (CD28, CTLA). The encounter between the dendritic cells containing HLA antigens and the T cell receptors is the key initiating step in cellular rejection. The T cell receptor contains an α- and a β-chain as well as CD3 chains. The CD3 chains are the targets for OKT3 (A). The combination of dendritic cells and T cell receptors is called the “immunologic synapse,” which consists of donor HLA antigens, recipient T cell receptors and CD 4 or CD 8 molecules, costimulatory molecules, and adhesion molecules. Belatacept blocks the costimulatory molecules (A).
Second, activation of T cells occurs via three intracellular signaling pathways for maturation of these cells: (1) calcineurin pathway; (2) mitogen-activated protein kinase pathway; and (3) JAK/STAT (Janus kinase/Signal Transducers and Activators of Transcription) pathway. The first two pathways cause the production of IL-2, which binds to its receptor (IL-2R) on T cells. This binding leads to the activation of the mammalian target of rapamycin (mTOR), which allows the T cells to undergo cell division.
Finally, the rejection of donor kidney is prevented by several immunosuppressive agents at several steps of T cell activation. Cyclosporine and tacrolimus inhibit the calcineurin pathway (B), daclizumab and basiliximab inhibit the interaction between IL-2 and its receptor (C), and sirolimus and everolimus inhibit mTOR, whereas mycophenolate mofetil and azathioprine inhibit cell division. More specifically, mycophenolate mofetil inhibits inosine monophosphate dehydrogenase, and this enzyme controls the synthesis of RNA. Azathioprine is a purine synthesis inhibitor, and purines are involved in the synthesis of RNA, DNA, ATP, etc. JAK3 inhibitors (CP-690 550) inhibit JAK/STAT pathway. The following diagram shows T cell activation by the donor dendritic cell and the inhibitory sites by various immunosuppressive agents (Fig. 11.2 ).
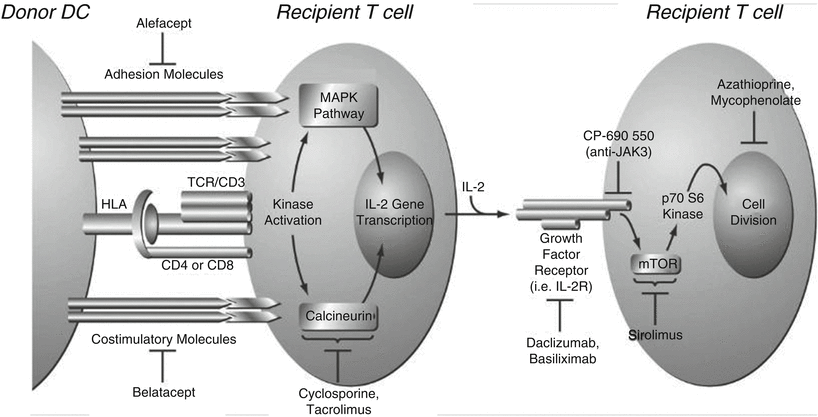

Fig. 11.2
T cell activation by the donor dendritic cell (DC) and the inhibitory sites by various immunosuppressive agents ((adapted from McKay DB, Steinberg SM, with permission from Springer)
Suggested Reading
McKay DB, Park K, Perkins. What is transplant immunology and why are allografts rejected? In McKay DB, Steinberg SM (eds). Kidney Transplantation. A Guide to the Care of Kidney Transplant Recipients. New York, Springer, pp 25–39.
Weltz A, Scalea J, Popescu M, et al. Mechanisms of immunosuppressive drugs . In Weir MR, Lerma EV (eds). Kidney Transplantation. Practical Guide to Management. New York, Springer, 2014, pp 127–141.
16.
Which one of the following drugs does NOT increase blood levels of calcineurin inhibitors (CNIs)?
A.
Ketoconazole
B.
Erythromycin
C.
Diltiazem
D.
Atorvastatin
E.
Caspofungin
The answer is E
CNIs include cyclosporine and tacrolimus, and are the most commonly used immunosuppressive agents. Both agents are metabolized by cytochrome P-450 (CYP450) enzyme system, and drugs that either induce or inhibit CYP450 may influence the levels of CNIs. Also, the levels of CNIs are influenced by a protein called P-glycoprotein. Except for caspofungin, all other drugs inhibit CYP450 and increase CNIs levels. Thus, option E is correct. The following table (Table 11.5 ) shows all the drugs that either increase or decrease blood CNIs levels.
Table 11.5
Effects of drugs on CNIs levels
Drugs that increase blood levels of CNIs by inhibition of CYP450 and/or P-glycoprotein | Drugs that decrease blood levels of CNIs by induction of CYP450 and/or P-glycoprotein |
Calcium channel blockers | Antituberculous drugs |
Dialtiazem, verapamil, amlodipine, | Rifampim, rifabutin, isoniazid |
nicardipine | Anticonvulsants |
Antifungal agents | Barbiturates, phynytoin, carbamazepine |
Ketoconozole, fluconazole, itraconazole, voriconazole | Antidepressive herbals |
Antibiotics | St. John’s wort |
Erythromycin, macrolides | Antibiotics |
Drugs/food | Nafcillin, imipenam, cephalosporins |
Metoclopramide, grape fruit juice | |
Statins | |
Protease inhibitors |
Suggested Reading
Chang PC-W, Hricik DE. What are immunosuppressive medications? How do they work? What are their side effects? In McKay DB, Steinberg SM (eds). Kidney Transplantation: A Guide to the Care of Kidney Transplant Recipients, New York, Springer, 2010; pp 119–135.
Danovitz GM. Immunosuppressive medications and protocols for kidney transplantation. In Danovitch GM (ed). Handbook of Kidney Transplantation, 5th ed, Philadelphia, Lippincott Williams & Wilkins, 2010, pp 77–126.
Kidney Transplantation- Principles and Practice. Morris PJ, Knechtle SJ (eds). Philadelphia, Elsevier Saunders, 7th ed, 2013.
17.
Nephrotoxicity is a well-known complication of calcineurin inhibitors (CNIs). Which one of the following renal-related complications due to CNIs is CORRECT?
A.
Hypertension
B.
Hyperkalemia
C.
Chronic kidney Disease (CKD)
D.
Hypomagnesemia, hypophosphatemia, type 4 renal tubular acidosis (RTA)
E.
All of the above
The answer is E
Both cyclosporine and tacrolimus cause nephrotoxicity. Hypertension is common in posttransplant patients receiving one of these drugs. The mechanism of hypertension includes endothelial dysfunction, decreased production of nitric oxide and other vasodilators with an increase in endothelin levels. These changes lead to renal and systemic vasoconstriction with resultant hypertension. Hyperkalemia is due mainly to inhibition of K + channel in the distal tubule, and decreased renin-AII-aldosterone activity. Type 4 RTA has also been reported, which is due to a decrease in NH 4 + synthesis by hyperkalemia. Hypomagnesemia and hypophosphatemia are due to renal loss of these electrolytes. Interestingly, long-term use of cyclosporine in patients with liver and heart transplantation can lead to CKD and progression to end-stage renal disease. Chronic tubulointerstitial disease, cytokine production (TGF-β1), and renal vasoconstriction seem to be responsible for CKD. Thus, option E is correct.
In addition to above changes, CNIs cause endothelial damage as well as renal vasoconstriction, resulting in thrombotic microangiopathy and renal ischemia.
Suggested Reading
Bennett WM, DeMattos A, Meyer MM, et al. Chronic cyclosporine nephropathy: The Achilles’ heel of immunosuppressive therapy. Kidney Int 50:1089–1100, 1996.
Lee CH, Kim G-H. Electrolyte and acid–base disturbances induced by calcineurin inhibitors. Electrolyte Blood Press 5:126–130, 2007.
Danovitz GM. Immunosuppressive medications and protocols for kidney transplantation. In Danovitch GM (ed). Handbook of Kidney Transplantation, 5th ed, Philadelphia, Lippincott Williams & Wilkins, 2010, pp 77–126.
18.
Many drugs exacerbate calcineurin inhibitor (CNI) nephrotoxicity. Which one of the following agents does NOT potentiate nephrotoxicity of CINs?
A.
Amphotericin
B.
Nonsteroidal anti-inflammatory drugs (NSAIDs)
C.
Aminoglycosides
D.
Sirolimus
E.
Prostacyclin E (PGE)
The answer is E
It is very important to recognize certain drugs that potentiate CNIs nephrotoxicity. These include amphotericin, NSAIDs, inappropriately high doses of aminoglycosides, and sirolimus. Also, concomitant use of ACE-Is or ARBs with CNIs requires close monitoring for hyperkalemia and acute kidney injury. P G E is a vasodilator, which has least interaction with CNIs. Thus, option E is correct.
Suggested Reading
Chang PC-W, Hricik DE. What are immunosuppressive medications? How do they work? What are their side effects? In McKay DB, Steinberg SM (eds). Kidney Transplantation: A Guide to the Care of Kidney Transplant Recipients, New York, Springer, 2010; pp 119–135.
Danovitz GM. Immunosuppressive medications and protocols for kidney transplantation. In Danovitch GM (ed). Handbook of Kidney Transplantation, 5th ed, Philadelphia, Lippincott Williams & Wilkins, 2010, pp 77–126.
Manitpisitkul W, Wilson NS, Lee S, et al. Drug interactions in solid organ transplant recipients. In Weir MR, Lerm EV (eds). Kidney Transplantation. Practical Guide to Management. New York, Springer, 2014, pp 411–425.
19.
A renal transplant patient is on cyclosporine and mycophenolate mofetil (MMF) for maintenance therapy. Which one of the following statements is FALSE regarding the use of MMF in this patient?
A.
MMF converts into active mycophenolic acid in the stomach
B.
MMF is a reversible inhibitor of inosine monophosphate dehydrogenase
C.
MMF is preferred to azathioprine as the drug of choice in the maintenance of immunosuppression in renal transplant patients
D.
Cyclosporine and tacrolimus equally lower MMF levels
E.
MMF is associated with increased teratogenicity in pregnancy
The answer is D
MMF and enteric-coated MMF are potentially useful antiproliferative immunosuppressive drugs with wide popularity. Both drugs are metabolized to mycophenolic acid. MMF requires acid environment for absorption, whereas enteric-coated MMF requires alkaline pH for absorption. Thus, MMF is absorbed in the stomach and enteric-coated MMF in the intestine. Both drugs are potent reversible inhibitors of inosine monophosphate dehydrogenase, which is the rate-limiting enzyme in the synthesis of guanosine monophosphate. Thus, these drugs inhibit the de novo synthesis of purines. Because of few side effects, well tolerability, and documented efficacy, MMF largely replaced azathioprine as the drug of choice in the maintenance phase of immunosuppression. Both animal and human studies have shown teratogenicity with MMF during pregnancy, and azathioprine is usually recommended until pregnancy is completed. Cyclosporine but not tacrolimus lowers serum levels of MMF. Thus, option D is false.
Suggested Reading
Chang PC-W, Hricik DE. What are immunosuppressive medications? How do they work? What are their side effects? In McKay DB, Steinberg SM (eds). Kidney Transplantation: A Guide to the Care of Kidney Transplant Recipients, New York, Springer, 2010; pp 119–135.
Danovitz GM. Immunosuppressive medications and protocols for kidney transplantation. In Danovitch GM (ed). Handbook of Kidney Transplantation, 5th ed, Philadelphia, Lippincott Williams & Wilkins, 2010, pp 77–126.
20.
Drug–drug interactions are clinically significant. Which one of the following drugs LOWERS plasma levels of sirolimus?
A.
Cyclosporine
B.
Diltiazem
C.
Fluconazole
D.
Erythromycin
E.
Rifabutin
The answer is E
Sirolimus is an antiproliferative agent, which is an mTOR (mammalian target of rapamycin) inhibitor. Another mTOR inhibitor is everolimus. Both of them are metabolized by P450 enzyme system. Thus, an interaction occurs when 2 P450 metabolizable drugs are used simultaneously. Because of this interaction, the primary drug’s levels may be either low or elevated. Except for rifabutin, all other drugs increase plasma levels of sirolimus by decreasing its metabolism. Rifabutin lowers sirolimus levels by increasing its (sirolimus) metabolism. When cyclosporine and sirolimus are used as immunosuppressive drugs, it is preferable to administer sirolimus 4 h after cyclosporine. Adverse effects of sirolimus include hyperlipidemia, anemia, and neutropenia.
Suggested Reading
Chang PC-W, Hricik DE. What are immunosuppressive medications? How do they work? What are their side effects? In McKay DB, Steinberg SM (eds). Kidney Transplantation: A Guide to the Care of Kidney Transplant Recipients, New York, Springer, 2010; pp 119–135.
Danovitz GM. Immunosuppressive medications and protocols for kidney transplantation. In Danovitch GM (ed). Handbook of Kidney Transplantation, 5th ed, Philadelphia, Lippincott Williams & Wilkins, 2010, pp 77–126.
21.
Which one of the following biologic immunosuppressive drugs does NOT deplete lymphocytes?
A.
Thymoglobulin
B.
Atgam
C.
Muromonab CD3 (OKT3)
D.
Basiliximab
E.
Alemtuzumab
The answer is D
Thymoglobulin and Atgam are polyclonal antibodies that are prepared by immunization of rabbit (Thymoglobulin) or horse (Atgam) against human lymphocytes. Atgam is largely replaced by Thymoglobulin. OKT3 is a monoclonal antibody against CD3 antigen associated with the T cell receptor. The use of OKT3 results in early activation of T cells with resultant release of cytokines followed by blocking T cell function. Alemtuzumab (Campath) is a monoclonal antibody against CD52, which was developed to treat chronic lymphocytic leukemia. Thus, thymoglobulin, atgam, OKT3, and alemtuzumab deplete both T and B lymphocytes, and thus are useful for induction of immunosuppression.
Basiliximab is a monoclonal CD25 antibody that binds to the α-chain of the IL-2 receptor activated cells. It is an IgG1 antibody and blocks the proliferative signal without depleting lymphocytes. Thus, option D is correct.
Suggested Reading
Chang PC-W, Hricik DE. What are immunosuppressive medications? How do they work? What are their side effects? In McKay DB, Steinberg SM (eds). Kidney Transplantation: A Guide to the Care of Kidney Transplant Recipients, New York, Springer, 2010; pp 119–135.
Danovitz GM. Immunosuppressive medications and protocols for kidney transplantation. In Danovitch GM (ed). Handbook of Kidney Transplantation, 5th ed, Philadelphia, Lippincott Williams & Wilkins, 2010, pp 77–126.
Kirk AD. Antilymphocyte globulin, monoclonal antibodies, and fusion proteins. In Morris PJ, Knechtle SJ (eds). Kidney Transplantation- Principles and Practice, 7th ed,. Philadelphia, Elsevier Saunders, 2013, pp 287–313.
22.
Match the following drugs with their mechanism of action in renal transplantation:
Drug | Mechanism |
|---|---|
A. Alemtuzumab | 1. Anti-CD20 antibody |
B. Rituximab | 2. Anti-CD52 antibody |
C. Intravenous immunoglobulin (IVIG) | 3. Proteosome inhibitor |
D. Bortezomib | 4. Anti-complement 5 antibody |
E. Eculizumab | 5. Reduction of alloantigen antibodies titers |
Answers: A = 2; B = A; C = 5; D = 3; E = 4
Alemtuzumab is a humanized IgG1 monoclonal antibody against CD52, which is present on cell surface of T and B lymphocytes, macrophages, monocytes, and granulocytes. It is approved for the treatment of B cell chronic lymphocytic leukemia. However, alemtuzumib is used in induction therapy of organ transplantation , which depletes peripheral and central lymphoid cells.
Rituximab is a monoclonal antibody that is directed against CD20 and IgG1 constant region. CD20 mediates B cell proliferation and differentiation, and rituximab inhibits these processes. B cells act as antigen presenting cells and activate T cells, and rituximab inhibits this T cell activation.
The function of IVIG in transplantation is to deplete alloantibody titers by inhibiting their production and increasing their catabolism.
Bortezomib is a proteosome inhibitor, causing cell cycle arrest and apoptosis. It causes thrombocytopenia and peripheral neuropathy. It is also used in desensitizing patient.
Eculizumab is a humanized monoclonal antibody directed against complement factor C5 and prevents its conversion into C5a and C5b. It is used in paroxysmal nocturnal hemoglobinuria and atypical HUS. Eculizumab is used for prevention of antibody-mediated rejection. Meningococcal infection is a serious complication of eculizumab treatment.
Suggested Reading
Chang PC-W, Hricik DE. What are immunosuppressive medications? How do they work? What are their side effects? In McKay DB, Steinberg SM (eds). Kidney Transplantation: A Guide to the Care of Kidney Transplant Recipients, New York, Springer, 2010; pp 119–135.
Danovitz GM. Immunosuppressive medications and protocols for kidney transplantation. In Danovitch GM (ed). Handbook of Kidney Transplantation, 5th ed, Philadelphia, Lippincott Williams & Wilkins, 2010, pp 77–126.
Bodell, Womer KL, Rabb H. Immunosuppressive medications in kidney transplantation. In Johnson RJ, Feehally J, Floege J (eds). Comprehensive Clinical Nephrology, 5th ed, Philadelphia, Elsevier/Saunders, 2015, pp 1144–1151.
23.
A 33-year-old African American male receives a deceased donor kidney transplant, and had antibody induction therapy. Which one of the following statements regarding induction therapy is CORRECT?
A.
African American race is a risk factor for acute rejection
B.
Rabbit antithymocyte globulin (rATG) is the most commonly used agent in induction therapy
C.
OKT3 is off-label since 2009 in the USA
D.
T cell depleting agents are associated with future development of lymphoma compared to nondepleting agents
E.
All of the above
The answer is E
It has become a common practice to use a brief course of immunosuppressive agents to prevent acute rejection in both high and low risk patients at the time of transplant. This strategy is called induction therapy. According to one source, 83 % of kidney transplant recipients received induction therapy in the USA in 2011. There are several risk factors for acute rejection. One of them that is still considered is the African American ethnicity, although a French study found no difference in rejection rates in European Africans and European Caucasians (A is correct).
Among polyclonal antibody preparations, rATG (thymoglobulin) is the most commonly used agent for induction therapy (B is correct). OKT3 is a monoclonal antibody that is directed against CD3 complex. It causes a “cytokine storm” that is potentially dangerous. Because of this profound cytokine release, OKT3 is withdrawn from market in 2009 with remaining stocks to be used up (C is correct).
Induction agents are classified as T cell depleting or nondepleting agents (see question 21). It has been shown that T cell depleting agents may predispose patients to the development of lymphomas compared to either nondepleting agents or no induction therapy (D is correct). The above (Table 11.6 ) summarizes the drugs that are used for induction therapy.
Table 11.6
Drugs for induction therapy
Drug | Action/effect | Dose |
|---|---|---|
Methylprednisolone | Inhibition of numerous cytokine production | 500 mg intravenously (iv) intraoperatively followed by tapering over 1–5 days |
Thymoglobulin (rATG) | Depletion of T lymphocytes | 1–1.5 mg/kg iv for 4–14 days for a total dose of 6 mg/kg |
Basiliximab | Inhibition of IL-2 receptor activity | 20 mg iv on 0 and 4 days |
Daclizumab | Inhibition of IL-2 receptor activity | 1–2 mg/kg iv every 2 weeks for 5 doses (withdrawn) |
Alemtuzumab | Anti-CD52 antibody | 30–60 mg 1 or 2 doses on days 0 and 4. (a single 30 mg intraoperative dose is commonly used in clinical practice) |
OKT3 | Blocks CD3 complex | 5 mg iv for 7–14 days (withdrawn) |
Suggested Reading
Hardinger KL, Brennan DC, Schnitzler MA. Rabbit antithymocyte globulin is more beneficial in standard kidney than in extended donor recipients. Transplantation 87:1372–1376, 2009.
Hanaway MJ, Woodle ES, Mulgoankar S, et al. For the INTAC Study Group. Alemtuzumab induction in renal transplantation. N Engl J Med 364:1909–1919, 2011.
Wiseman AC, Cooper JE. Prophylaxis and treatment of kidney transplant rejection. In Johnson RJ, Feehally J, Floege J (eds). Comprehensive Clinical Nephrology, 5th ed, Philadelphia, Elsevier/Saunders, 2015, pp 1176–1187.
24.
A 52-year-old woman on hemodialysis is called for kidney transplantation from a 60-year-old deceased donor. She was explained the medical history of hypertension, smoking, alcoholism, and prostatism of the donor. She is curious about transmission of infection and malignancy from the donor. Which one of the following statements regarding transmission of malignancy from the donor history of smoking and alcoholism and transmission of infection from prostatism is CORRECT?
A.
Renal cell carcinoma accounts for most of the donor-derived malignancy
B.
Donor transmission rate of renal carcinoma is 0.1 %
C.
Donor-derived transmission rate of infection is <1 %
D.
Predonor testing for HIV, hepatitis B, and hepatitis C may be negative during “window” period
E.
All of the above
The answer is E
A survey from 1994 to 2001 identified a total of 21 donor-related malignancies from 14 cadaveric and three living donors were identified. Fifteen tumors were donor-transmitted (malignancies that were present in the donor at time of transplantation) and six were donor-derived (de novo tumors that developed after transplantation). The cadaveric donor-related tumor rate was 0.04 %.
Between 2005 and 2009, a total of 146 cases of donor-derived malignancies were reported in the USA. Of these, 64 cases (43.8 %) were renal cell carcinomas, and only 7 of 64 (0.1 %) were related to donor transmission cancers (A, B are correct). Also, donor-derived transmission of infection rate was <1 % during 2005 to 2009 years (C is correct).
Donors are routinely screened for HIV, hepatitis B, and hepatitis C. However, these tests may be negative during the “window period,” a period between infection and detection by routine tests. Thus, transmission of these viruses may be missing at time of donation or removal of the kidney from a deceased donor (D is correct).
Suggested Reading
Ison MG, Nalesnik MA. An update on donor-derived disease transmission in organ transplantation. Am J Transplant 11:1123–1130, 2011.
Desai R, Collett D, Watson CJ, et al. Cancer transmission in solid organ donors-an unavoidable but low risk. Transplantation 94:1200–1207, 2012.
26.
Which one of the following statements regarding the epidemiology of malignancy in kidney transplant recipients is CORRECT?
A.
Skin cancers are the most common de novo cancers in the adult transplant patients that occur 20–30 years earlier in immunosuppressive patients as compared with general population
B.
Posttransplant lymphoproliferative disorders (PTLDs ) are the most common nonskin malignant disorders in kidney recipients
C.
In patients with a history of preexisting malignant neoplasms, close monitoring for recurrence is highly recommended
D.
Among malignancies, recurrence rates of multiple myeloma are much higher than those of nonmelanoma skin and other cancers
E.
All of the above
The answer is E
All of the statements are correct (E). In 2004, Kasiske et al. reported the rates of malignancies in a large number of first time recipients of either deceased or living donor kidney transplantations between 1995 and 2001 using Medicare billing claims. A twofold higher rate for cancers such as colon, lung, prostate, stomach, esophagus, pancreas, ovary and breast, cancer rates was reported after kidney transplantation as compared with the general population. A fivefold higher rates for melanoma, leukemia, hepatobiliary tumors, cervical and vulvovaginal tumors, a threefold higher rates for testicular and bladder cancers, a 15-fold higher rates for kidney cancers, and >20-fold for Kaposi’s sarcoma, non-Hodgkin’s lymphomas, and nonmelanoma skin cancers. The authors concluded that the rates for most malignancies are higher after kidney transplantation as compared with the general population.
Among all cancers, skin cancers are the most common de novo posttransplant cancers in adults and their occurrence increases with time. Among nonskin malignant cancers, PTLDs are the most common type of tumors. Cancers such as Kaposi sarcoma, PTLDs, testicular cancer, cancer of the small intestine, and thyroid occur before 800 days after transplantation
In patients with a history of preexisting malignant neoplasms, close monitoring for recurrences after kidney transplantation is highly recommended. Among malignancies, the recurrence rate for multiple myeloma is 67 % followed by 53 % for nonmelanoma skin cancers, 29 % for bladder cancers, 29 % for sarcomas, 27 % for symptomatic renal cell carcinomas, and 23 % for breast carcinomas. The recurrence of prostate cancer is dependent on the stage of the disease, with highest rate for stage III disease. Thus, cancer screening is mandatory for recurrent disease after kidney transplantation.
Suggested Reading
Penn I. Evaluation of transplant candidates with pre-existing malignancies. Ann Transplant. 2:4-17, 1997.
Kasiske BL, Snyder JJ, Gilbertson DT, et al. Cancer after kidney transplantation in the United States. Am J Transplant 4:905–913, 2004.
Sampaio MS, Cho YW, Qazi Y, et al.: Posttransplant malignancies in solid organ recipients: An analysis of the U.S. National Transplant database. Transplantation 94:990–998, 2012.
Tessari G, Nadi L, Boschiero L, et al.: Incidence of primary and second cancers in renal transplant recipients: A multicenter cohort study. Am J Transplant 13:214–221, 2013.
Pham P-T, Danovitz DM, Pham P-CT. Medical management of the kidney transplant recipient: Infections, malignant neoplasma, and gastrointestinal disorders. In Johnson RJ, Feehally J, Floege J (eds). Comprehensive Clinical Nephrology, 5th ed, Philadelphia, Elsevier/Saunders, 2015, pp 1188–1201.
27.
De novo development of malignancy is common in recipients of solid organ transplantation. Which one of the following choices regarding the mechanisms of malignancy after transplantation is CORRECT?
A.
Immunosuppression
B.
Antigenic stimulation in the presence of immunosuppression
C.
Neoplastic activity of immunosuppressive drugs
D.
Increased susceptibility to oncogenic viral infection
E.
All of the above
The answer is E
All of the above choices are correct (E). Immunosuppressive drugs may alter the tumor surveillance by affecting both innate and adaptive immunity. This causes potentially malignant cells to be seeded in the host and escape usual killing by the immune system. Also, the foreign HLA antigens in the allograft may stimulate its own lymphoreticular system in the development of lymphomas. In addition, immunosuppressive drugs themselves may deplete T cells, and promote the development of posttransplant lymphoproliferative disorders (PTLDs) following induction therapy. Both cyclosporine and tacrolimus have been shown to cause higher rates of malignancy by stimulating transforming growth factor-β. Belatacept, an inhibitor of costimulatory signals, is associated with higher incidence of PTLDs, particularly in EBV seronegative recipients who received kidneys from EBV seropositive donors. Similarly, azathioprine, an antimetabolite, may sensitize skin to UV light and promote skin cancer. Finally, immunosuppressive therapy may activate latent oncogenic viruses (EBV, herpes virus, papilloma virus, hepatitis B and C viruses), which promote the development of malignancy. In addition, preexisting cancers that were undetectable at time of transplantation may be activated by any one of the above mechanisms. Thus, E is correct.




Suggested Reading
< div class='tao-gold-member'>
Only gold members can continue reading. Log In or Register to continue
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree






