Fig. 8.1
Mechanisms claimed for TENS effectiveness
TENS-induced activity in Aδ and C afferents has also been shown to produce extra-segmental analgesia through the activation of structures which form the descending pain inhibitory pathways, such as periaqueductal gray (PAG), nucleus raphe magnus, and nucleus raphe gigantocellularis.
Furthermore this type of TENS activates opioid receptors located peripherally, in the spinal cord and in areas involved in descending inhibition including the nucleus raphe magnus in the rostral ventral medulla and the PAG [5].
The normal function of the pelvic floor muscles is essential for supporting the pelvic visceras and maintaining urinary and fecal continence.
The etiology of urinary incontinence is multifactorial with the most common cause being dysfunctions of the pelvic floor muscles. Therefore, weakened pelvic floor muscles may be assumed to predispose women to an increased risk of developing urinary continence.
TENS aims to strengthen the pelvic floor muscles in an attempt to recover urinary continence mechanisms.
The effect of electrical stimulation on pudendal afferents in the treatment of urinary incontinence has been described and can be reassumed as follow [6]:
Reflex activation of sympathetic inhibitory neurones
Reflex central inhibition of parasympathetic excitatory neurons
TENS can be used in the treatment of fecal incontinence. It is postulated that stimulation of the skin in the distribution of the S3 dermatome excites mainly the Aβ fibers, thereby modulating signals to and from the pelvic organs with a possible effect on continence.
Essentially, there are four broad categories of anatomical site to which TENS electrodes can be applied: painful area, peripheral nerve, spinal nerve roots, and other specific points (acupuncture, trigger, and motor points). Irrespective of the electrode site that is chosen, stimulation will ultimately result in the passage of afferent information into the central nervous system [7].
Vaginal electrodes are the most commonly used devices for applying electrical currents for pelvic floor muscle problems in women (Fig. 8.2). The vagina offers a route of low impedance, because of the low resistance of the vaginal mucosa and proximity to branches of the pudendal nerves.
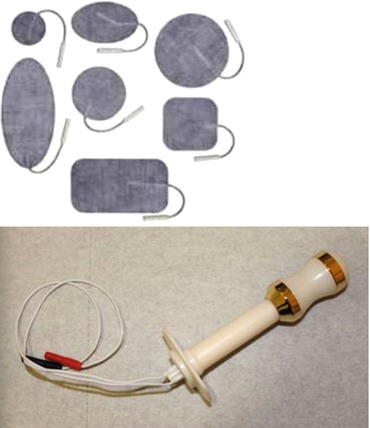

Fig. 8.2
TENS electrodes: patches and vaginal probes
Anal electrodes are suitable for patients with anal sphincter weakness. Anal electrodes can also be used in narrow vaginas, where a vaginal electrode is too big and painful to insert. Electrodes suitable for electrical stimulation may also be used for electromyography (EMG) biofeedback.
8.3 TENS Parameters
The stimulation parameters that are set on the TENS unit determine the type of nerve fibers stimulated and thus its mechanism of action.
8.3.1 Frequency
The frequency of a current refers to the number of pulses delivered per second; therefore, a frequency of 200 Hz means that 200 pulses are delivered per second.
Both high (50–100 Hz) and low (5–10 Hz) frequencies can be applied and are thought to work by different mechanisms. High-frequency TENS is thought to selectively activate large-diameter non-noxious Aβ afferents to reduce nociceptor cell activity and sensitization at a segmental level in the central nervous system [8]. Low-frequency TENS can be applied and activates small diameter motor afferents to elicit extra-segmental analgesia [8].
Concentrations of β-endorphins increase in the bloodstream and cerebrospinal fluid of healthy subjects after administration of either high- or low-frequency TENS. This suggests that at the spinal level there are different opioids released with different stimulation frequencies and thus possibly different opioid receptors activated to produce analgesia with high- or low-frequency TENS [9].
Low-frequency TENS activates μ-opioid receptors in the spinal cord and the brainstem, whereas high-frequency TENS activates δ-opioid receptors in the spinal cord and the brainstem [9].
It is also generally thought that large-diameter fibers are activated by high-frequency TENS and that low-frequency TENS at motor intensity activates Aδ afferent fibers [8].
Furthermore, basic science studies show that simultaneous activation of μ-opioid and δ-opioid receptors prevents the development of tolerance. Thus, providing low- and high-frequency TENS simultaneously, to activate μ-opioid and δ-opioid receptors, should similarly prevent tolerance to TENS [8].
8.3.2 Pulse Duration
The unit of pulse duration is usually given in microseconds (μs) which are units of time; hence, it is more correct to use the term “duration” rather than “width.” The pulse duration is usually defined as the duration of only the positive component of the waveform. TENS pulse durations are in the μs range (1 μs = 1 × 10−6 s).
Pulse duration currents of 30–100 μs activate large-diameter fibers without activating smaller nociceptive fibers. Wider-intensity pulses (around 100 μs) simultaneously stimulate “sensitive” and “pain” fibers. In this case, analgesic action is not derived from a gate control mechanism, but from the activation of the descending pain inhibitory pathways [10].
A pulse duration of 50–100 μs is well within the range which is expected to activate Aβ fibers.
The recommended pulse width for muscle stimulation is approximately 250, and 500 μs.
These pulse width leads to conversion of fast to slow twitch muscle fibers with the results in a muscle which is less susceptible to fatigue [6].
8.3.3 Duty Cycle
The duty cycle is the time the current is on and off. Typically, most TENS units allow the user to choose between continuous, burst, and modulated outputs. If the output is set for amplitude modulation, a cyclic modulation in amplitude is produced which increases from zero to a preset level and then back to zero again. The modulated output has been demonstrated to overcome accommodation of nerve fibers, hence providing more comfort to the patient [11].
8.3.4 Stimulation Intensity
Intensity refers to the magnitude of current or voltage applied by the TENS unit. Aarskog et al. [12] used pressure pain threshold (PPT) to compare two intensity levels of high-frequency TENS (100 Hz) applied simultaneously for 20 min to the hand/forearm on both sides. The intensity levels were either the lowest intensity at which the participant first perceived the electrical stimulation on the skin (sensory threshold) or at a level that the participant described as strong but comfortable. There was a statistically significant increase in PPT on the strong-but-comfortable intensity side but not on the sensory-threshold intensity side.
The relevance of stimulus intensity was also highlighted in a study by Claydon et al. [13].
These authors found that TENS at a high intensity (to tolerance) using different frequencies at each site produced the greatest hypoalgesia. These results indicate that the high-intensity currents (irrespective of the applied frequency) are the key parameter in TENS applications.
Sjolund found that stimulation at high frequency (80 Hz) and high intensity (10× sensory threshold) of the plantar and sural nerves produced maximal suppression of the C fiber-evoked flexion response in a rat [14].
In the TENS treatment protocol, the pulse has to be increased rapidly until the patient reports the onset of any sensation under the electrodes. The intensity is then increased slowly until this sensation reaches a level described as the maximum tolerable, without experiencing pain.
8.4 TENS and Vulvar Pain
Vulvodynia is a complex, common, and multifactorial condition manifesting pain in the vulvar area with an estimated prevalence of up to 16 % [15].
The disease, most often described as a burning pain, occurring in the absence of relevant visible findings or a specific, clinically identifiable, neurological disorder. It is classified according to the site of the pain, whether generalized or localized and whether provoked, unprovoked, or mixed. Vestibulodynia defines the most common localization, which is at the vulval vestibule [16]. Introital dyspareunia, the intensity of which may inhibit or prevent intercourse, is often the presenting symptom.
The etiology of vulvodynia is not fully understood. Many findings suggest that neuropathic mechanisms may underlie the clinical symptoms of the disease including neural hyperplasia, inflammation, central or peripheral nociceptive dysfunction, and involvement of contiguous pelvic floor muscles [17].
Central and peripheral sensitization seems to be responsible for perpetuation of the symptoms long after any “triggering factor” (infections, trauma, allergy, hormonal factors,etc.) has been resolved.
These sensitized afferent nerve fibers discharge more readily and at lower thresholds, helping to explain why apparently imperceptible or minimal stimulation sometimes causes pain [17].
In light of the complex neuropathology of this syndrome, an effective therapeutic approach should target both peripheral and central neural sensitization.
It has been demonstrated that TENS is of significant benefit in the management of vestibulodynia, in fact it provides a therapeutic neuromodulation based on presynaptic inhibition in the dorsal horn of the spinal cord; moreover it acts on direct inhibition of an abnormally excited nerve and on restoration of afferent input explained by the “gate control theory” [17].
Contextually, electrical stimulation delivered by a TENS unit activates sovraspinal inhibitory systems and it increases the release of endogenous morphine-similar substances (amplification of endogenous descending system for analgesia), but it is essential to use appropriate and validated stimulation parameters [17].
Electroanalgesia with diphasic currents of frequencies between 2 and 100 Hz and 50–100 μs pulse duration has been used in the treatment of localized vulvodynia, with a high improvement (75 %) superior to placebo [3]. In all trials using TENS in vulvodynia, the stimulation was delivered through plastic vaginal probe inserted in into the vagina for 20 mm.
The number of treatment sessions and interval between sessions are also worth discussion.
A daily regimen consisting of a period of 2 weeks of daily TENS applications (10–14 sessions) was considered appropriate to assess the efficacy of the treatment [18].
We think that a twice-weekly or an alternate-day regimen was preferred to the commonly reported daily regimen in relation to the site of application of the TENS electrode.
In fact, the vagina is more delicate and thinner than the skin, which is the site where TENS was applied in many reports. Irritation or reddening beneath or around the TENS probe may result in areas of broken or damaged skin. A twice-weekly or alternate-day regimen were also preferred to a daily treatment to minimize for a possible decline in response due to tolerance to TENS analgesia [5].
This effect has been interpreted as an adaptive change by the nervous system to the TENS regular repetitive stimuli.
TENS treatment can also be self-administered in the privacy of a woman’s home after a short period of supervision, using an inexpensive device. Our experience with a large series of provoked vestibulodynia patients (480 women) showed that there is a positive response after 10–15 sessions (symptom reduction >50 %) which tends to peak after 25–35 sessions [17].
Furthermore, we observed that nociceptive system is best suited to the new situation through a gradual increasing of day numbers between TENS sessions.
Pelvic floor hypertonic dysfunction is found in 80–90 % of patients with vulvodynia [19].
The leading opinion indicates that vulvar pain can produce spasm of the levator ani muscle, and pelvic floor hypertonicity contributes to self-maintenance of pain.
It is not important what starts the process (muscle or nerve), but it is important how alteration of the pelvic muscles is responsible for the severity of symptoms. Indeed, “the weight of the muscle” may be different between patients with vulvodynia, and this is the only important target of the treatment program.
TENS can also help to reduce the pelvic floor hypertonicity working through the following two main modes:
Directly with an electrical muscle stimulation, in fact the levator ani muscles are innervated by the levator ani nerve, while no evidence of innervation by the pudendal nerve can be found. The levator ani motor neurons are diffusely distributed in the sacral ventral horn, while the pudendal motor neurons are concentrated in Onuf’s nucleus (a group of neurons located in the ventral part of laminae IX of the anterior horn). However, there is a great deal of overlap between the dendrites of levator ani motor neurons and pudendal motor neurons, and both nerves contain primary afferent fibers that project into the sacral spinal cord [20]. Thus, there is great potential for interaction between the sensory and motor nerve fibers that control the levator ani muscle, the vulva, and the vestibule.
Indirectly because TENS reduce the vulvar pain with the results in a secondary decrease in muscle activity and spasm. One of the more common neuropathic reflexes that occur is visceromuscular hyperalgesia. This results in muscular instability and a hypertonic contractile state within the muscles of the pelvic floor.
The ideal approach of vulvodynia, however, is a multimodal interventions with the use of more than one type of therapy for the care of patients with chronic pain.
Physical therapy is an important complement to any therapy for the vulvodynia associated with levator ani hypertone and myalgia.
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree





