Author (reference)
Country
Incidence
Percentage
Iannettoni [6]
USA
1/856
0.12
Bartels [7]
Germany
4/501
0.80
Buskens [5]
Netherlands
1/383
0.26
Maruyama [8]
Japan
2/305
0.66
Yasuda [9]
Japan
9/603
1.49
Schweigert [10]
Germany
7/222
3.15
Kuwabara [11]
Japan
9/475
1.89
Total
33/3345
0.99
Clinical Presentation
There are a number of signs and symptoms of TEF which are related to the size, location, and stage of the TEF. Early and small fistulas may simply present with cough after oral ingestion, also known as Ono’s sign. Persistent airway soilage typically leads to pneumonia, and signs of sepsis and respiratory insufficiency follow. In PETEF developing after an anastomotic leak, there may be accompanying mediastinal sepsis, and patients are usually critically ill with multiorgan dysfunction.
If a TEF develops in a mechanically ventilated patient, there is usually a sudden increase in airway secretions, which represents contamination with saliva or gastric contents. It may be difficult to maintain a seal with the endotracheal tube’s cuff, and in extreme cases, ventilation may become impossible if the tip of the tube migrates into the fistula. Positive pressure ventilation may lead to air leakage into the esophagus or gastric conduit and leads to abdominal distention or air escaping the pharynx.
Diagnosis
A chest x-ray may find a dilated esophagus or gastric conduit secondary to air leakage through the TEF. Computed tomography (CT) delineates the fistula with good detail in large or giant TEFs, which are defined as a fistula involving the entire width of the membranous wall. The CT may also identify anastomotic or conduit disruption after esophagectomy and also accurately reveals mediastinal and pleural collections.
Contrast esophagography has a role in milder presentations of TEF, when patients are able to participate in a swallow study. Water soluble contrast agents are strictly avoided as they can severely exacerbate pulmonary injury. Barium is typically used, and contrast outlining the trachea or bronchus is seen. An experienced radiologist is able to localize the level of fistula with respect to the airway and the esophagus (or neo-esophagus).
Endoscopic inspection of the tracheobronchial tree and esophagus further elucidate the location and nature of the fistula. While a small fistula may be difficult to appreciate in the folded mucosa of the esophagus or gastric conduit at esophagoscopy, it is usually apparent at bronchoscopy. In mechanically ventilated patients, the orotracheal or tracheostomy tube may need to be withdrawn to reveal the fistula. Esophagoscopy is useful to assess the integrity of an esophagogastric anastomosis and viability of a gastric conduit in patients with PETEF.
Management
Effective management of TEF requires a combination of conservative, endoscopic, and operative measures. The therapies chosen are predicated on the patient’s presentation and condition.
Conservative Management
When a patient presents early after a small TEF develops, the only complaint may be a cough with oral ingestion. Even before the diagnosis is confirmed, the patient is made strict nil per os. The patient is instructed to stay upright at all times, which minimizes reflux and ensures drainage of the gastric conduit in patients presenting after esophagectomy. When there are signs of tracheobronchitis or early pneumonia there is a low threshold to start empiric antibiotic therapy.
A more severe presentation of TEF is the patient with advanced pneumonia and frank respiratory failure. Mechanical ventilation is unavoidable in this situation. It is important to position endotracheal tubes with the cuff inflated beyond the location of the tracheal fistula, if possible. Bronchoscopic guidance of ortracheal and tracheostomy tubes is invaluable in these circumstances. Imprecise positioning can lead to exacerbation of the fistula, if the balloon is inflated adjacent to or within the fistula. When initially intubating the patient with a TEF, it is best to guide the tracheal tube over a bronchoscope, in order to avoid intubation of the fistula, a life-threatening event if it is not recognized immediately.
Even with the cuff positioned and inflated beyond the TEF, airway contamination is possible. Appropriate measures to decompress the stomach or gastric conduit are indicated to prevent ongoing soilage across the fistula. An aggressive pulmonary toilet with bronchoscopy and appropriate antibiotic therapy are the mainstays of treating pneumonia after the development of TEF. Weaning from positive pressure ventilation remains a priority and greatly facilitates the medical and surgical management of patients with TEF, as emphasized in the section on operative techniques.
Especially in the mechanically ventilated patient with a TEF, there is early consideration of jejunostomy tube placement to provide adequate enteral nutrition. A gastrostomy may also be considered to prevent reflux of gastric contents into the TEF.
There are a few reports of spontaneous closure of TEF with conservative management alone [14]. Only early and the tiniest of fistulas are expected to heal without operative management. These patients presumably had fistula tracts that had not already epithelialized, and ongoing drainage across the fistula was minimal. The fistula tracts that spontaneously close are usually long and likely lead into pulmonary parenchyma rather than the trachea or main-stem bronchus. Such patients are not ill, and a trial of conservative management is reasonable, as long as patients are closely observed for deterioration. In the vast majority of patients presenting with clinically significant TEFs, conservative management is expected to fail in the long-term.
Endoscopic Management
An increasing experience with esophageal and airway stents has led to their application in the management of anastomotic leaks and TEF. Exclusion of the fistula by covered stents may partially or completely control exchange of air and fluid across the fistula. There are isolated reports of acquired TEFs resolving after stenting [10]. As with the patients that had TEFs resolve with conservative management alone, stents are likely associated with fistula closure only when the TEF is extremely small and the tract is still not epithelialized, which is most commonly not the case. More typical of expected outcomes are the experiences of Blackmon et al., who placed stents to control the TEF in four patients with two patients succumbing to their TEF related medical problems and two reported to have control of the fistula without evidence of healing [15]. Even more concerning are the outcomes of Eleftheriadis et al., who used stents in 12 patients with TEF, observed nine deaths, and had 3 patients who went on to definitive operative management, as the TEF persisted after the stent placement [16].
Esophageal stents may actually potentiate the TEF-associated pathology. In one report, giant TEFs were induced by esophageal stents placed for a benign stricture or esophageal perforation [17]. The radial force of self-expanding esophageal stents has the potential to enlarge the TEF or exclude abscesses that would normally drain back into the esophagus. Another concern is that stenting does not address mediastinal sepsis that may accompany anastomotic disruptions or gastric conduit necrosis. Persistent mediastinal contamination and inflammation not only leads to TEF, but may also result in aortoesophageal fistula, which is almost uniformly fatal. One report, in which a silicone airway stent controlled a PETEF, describes a patient who eventually succumbed to hemorrhage that appeared suspicious for aortogastric fistula [18]. A further concern with airway stenting is that it induces inflammation and granulation. This may extend the length of airway injury, which complicates or precludes definitive operative repair. A technical difficulty with esophageal stent deployment for the PETEF is that there is only a limited esophageal length to accommodate the stent after a cervical anastomosis. Additionally, the anastomosis, conduit, and esophagus are relatively capacious relative to the stent’s diameter, and stent migration and poor sealing of the fistula are common.
There is very little role for esophageal or airway stenting to control the benign TEF. Conservative measures such as careful positioning of an endotracheal tube’s cuff, gastric decompression, and jejunal feeding are sufficient to allow a patient to recover from complications of a TEF prior to operative repair. Moreover, in patients with evidence of conduit necrosis and significant mediastinal or pleural contamination after an esophagectomy, stenting is absolutely contraindicated, and is expected to fail quite quickly. In contradistinction, esophageal stenting is the standard of care for the management of malignant TEF, and is quite effective in controlling the TEF during the short life-expectancy of such patients [19].
An alternative endoscopic strategy that is sometimes promoted is fistula control with glue or endoscopically applied clips. This strategy is most effective in pediatric cases of benign TEF, where fistulas are typically pinpoint and there is minimal associated pathology in the esophagus, airway, and mediastinum. Fistula closure is achieved by deepithelializing the fistula tract and sealing the defect with glue or clips [20]. Efficacy in adult cases of TEF is anecdotal and there is no reliable data to suggest that there is a role for endoscopically applied clips or glue in the management of acquired TEF, such as those that occur postesophagectomy.
Operative Management
Operative repair of an acquired TEF is indicated in all patients with a reasonable life expectancy. This includes patients who have undergone complete resection of esophageal cancer and develop PETEF. The patient is weaned from mechanical ventilation, as tracheal repairs should ideally not be exposed to positive pressure ventilation. Aggressive pulmonary toilet, appropriate antibiotic therapy, and reliable enteral nutrition are essential for the patient’s recovery. This may require tracheostomy and feeding tube placement if the patient does not quickly improve after presentation. The operative techniques are selected based upon the location and size of the TEF as well as the associated pathology (e.g., conduit necrosis).
Postesophagectomy TEF
PETEF occurs primarily after an anastomotic leak or gastric conduit necrosis. Patients are quite ill from pulmonary, mediastinal, and pleural contamination. Early operative intervention is typically necessary in these patients. Fistulas are predominantly located in the distal half of the trachea or proximal main-stem bronchus, but may be located more proximally if the anastomosis was constructed close to the cricopharyngeus. Preoperative endoscopy localizes the TEF and guides the surgeon as to whether the fistula may be approached with a low cervical collar incision or by thoracotomy. Endoscopy also establishes whether or not the conduit is ischemic. Flexible and rigid bronchoscopy determine whether there is any tracheal stenosis and to measure the distance of the fistula from the larynx and carina.
When conduit necrosis or major anastomotic dehiscence results in a TEF several days after an esophagectomy, and patients are critically ill, the appropriate operation is trans-thoracic takedown of the anastomosis. Nonviable stomach is resected, and the remainder is returned to the abdomen. The tracheal or bronchial defect is repaired primarily with interrupted vicryl suture, which minimizes airway granulation. The defect is buttressed with robust vascularized tissue, such as an intercostal muscle flap. The proximal esophagus is used to construct an esophagostomy, preserving as much esophagus as possible to facilitate future reconstruction. Thorough irrigation and drainage of the mediastinum and pleura, including decortication of the lung, is essential.
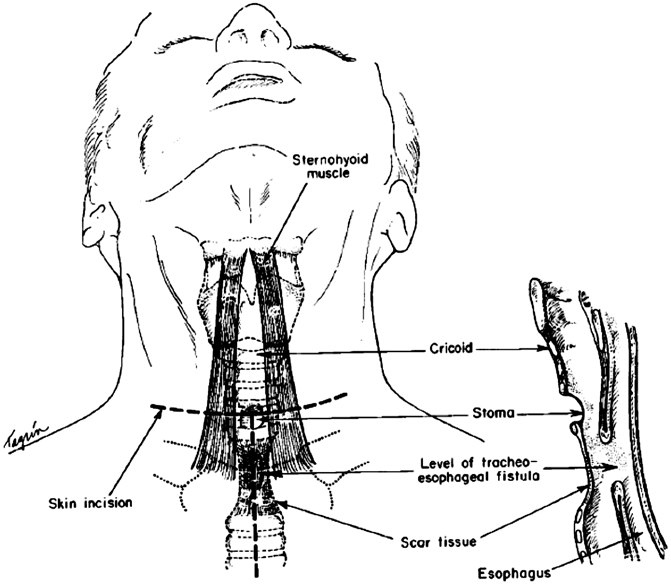
A TEF that occurs months to years after an esophagectomy is quite different in terms of presentation and pathology. There is minimal or no mediastinal inflammation and contamination. The conduit is viable and the anastomosis may be completely healed and intact. A more measured approach to operative repair may be taken and the patient’s condition is optimized with simple conservative measures. It is important to determine whether or not there is tracheal stenosis in addition to the TEF, as this will dictate whether or not a simple fistula division is all that is required or if a tracheal resection and reconstruction is necessary to address a significant stricture. All but the lowest supracarinal TEFs may be approached via a low cervical collar incision (Fig. 1.1). In TEF patients with a small fistula and normal trachea, the fistula is approached from the side, through the cervical incision (Fig. 1.2). The recurrent nerve on the side the fistula is approached from is at great risk, and care should be taken to avoid retractor injury or inadvertent division. Once the fistula is isolated and divided, the trachea is repaired with interrupted absorbable vicryl sutures. The esophageal or gastric conduit defect is repaired with a two-layered closure whenever possible. The inner layer is an interrupted inverted silk closure. A second outer layer is constructed with interrupted silk sutures approximating esophageal muscle or gastric serosa. A pedicled strap muscle is sutured in place to buttress and isolate the esophageal and tracheal suture lines, which otherwise would lie next to each other and predispose to fistula recurrence. When there is a relatively large defect in the membranous wall of the trachea and there is a concern of airway narrowing with primary repair, a small amount of esophageal wall may be left behind on the tracheal aspect of the fistula to augment the amount of tissue available to reconstruct the membranous wall. There is little concern about narrowing the lumen of the esophagus with this maneuver, as long as the residual lumen easily accommodates a nasogastric tube. Patients are extubated in the operating room whenever possible. A contrast esophagogram is performed after 7 days to ensure healing before starting oral alimentation.
 < div class='tao-gold-member'>
< div class='tao-gold-member'>





Only gold members can continue reading. Log In or Register to continue
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree






