Fig. 9.1
Urgent PC Neuromodulation System (Courtesy of Uroplasty, Inc., Minnetonka, MN)
9.2 PTNS Technique
The Stoller technique (SANS protocol) consists in the electrical stimulation of the posterior tibial nerve by a fine metallic needle 34 gauge inserted into the lower part of the leg, 3–5 cm cephalad to the medial malleolus [2]. Interestingly, the place where the needle is inserted had been already known by traditional Chinese acupuncture as the Sanyinjiao (SP6) point for the pelvic floor organ dysfunctions [8–10]. Patients lie supine with the soles of the feet together and their knees abducted and flexed (“frog position”). A grounding pad is placed in the medial face of the ipsilateral calcaneus. The needle electrode is then connected to an external low-voltage (9 V) pulse generator which delivers the electrical pulse (Fig. 9.2).
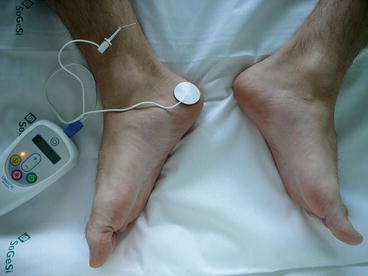

Fig. 9.2
Needle and pad positions
Once the electrode needle is correctly placed and the electrical impulse is on, the motor response consists in an involuntary toe flex or an extension of the entire foot while the sensory response results in a sensation in the ankle area or across the sole of the foot. The toe flex is obtained by a direct stimulation (retrograde or afferent) of the S3 nerve root, mostly responsible for the bladder innervation: this confirms the presence of S3 fibers in the tibial nerve.
A current level of 0.5–9 mA at 20 Hz at a fixed frequency of 20 Hz and pulse width of 200 μs is selected based on the subject’s motor and/or sensory responses. Each session lasts 30 min, and it’s repeated for 10–12 times usually on a weekly basis. Some authors have investigated more frequently stimulation or longer lasting session, reporting the advantage to obtain the same results in less time [11, 12].
Daily treatment may be more effective than twice weekly treatment [13].
PTNS is a low-risk procedure: only minor bleeding, mild pain, and skin inflammation resulting from the placement of the needle are reported [14].
On the basis of McGuire, transcutaneous stimulation of the tibial nerve (TTNS) using adhesive electrodes has also been demonstrated to be effective for either urinary and bowel dysfunction. However, it has been suggested that PTNS is more effective than surface stimulation probably because the needle electrode is closer to the tibial nerve [15].
In the study of George Percutaneous, transcutaneous and sham transcutaneous posterior tibial nerve stimulation was compared in a prospective blinded randomized placebo-controlled trial. Patients undergoing percutaneous nerve stimulation had a greater reduction in the number of incontinence episodes and were able to defer defecation for a longer interval than those undergoing transcutaneous and sham stimulation. These improvements were maintained over a 6-month follow-up period [16].
Adequately powered RCTs of PTNS vs TTNS stimulation are necessary to establish the short- and long-term effects on both techniques.
9.3 Results
9.3.1 The Urological Experience
9.3.1.1 Overactive Bladder (OAB) Syndrome
Over the last decades, several case studies have been published about the use of PTNS for the treatment of OAB syndrome. There is evidence that PTNS significantly improves OAB symptoms such as urinary frequency, urgency, and urgency urinary incontinence with a positive impact on QoL [17]. The percentage of success, in patient’s refractory to previous conservative treatments, after a PTNS round is about 60–80 % [18]. A multicenter double-blind controlled prospective study (SumiT) compared the efficacy of the active (54.5 %) and the sham therapy (20.9 %) [19]. Another multicenter RCT documented comparable efficacy of PTNS vs drug therapy (OrBiT trial). At 3 months, 79.5 % of the patients after PTNS vs 54.8 % of patients on tolterodine were improved [20]. Further the clinical results, urodynamic data were also reported by some authors [21–23]. According to Vandonick et al., the absence or the presence of a mild detrusor overactivity with normal bladder capacity seems to be a possible predictive parameter of success [23].
PTNS seems to be effective even in childhood. Hoebeke et al. reported a significant reduction (60 %) of LUTS, and 17 % resulted dry after treatment. These data have been confirmed by De Gennaro et al. observing an improvement of OAB symptoms in 80 % of children with a good acceptance to treatment and tolerability assessed by VAS scale. Moreover, considering the urodynamic outcomes, a normalization of cystometric capacity was seen in 62.5 % of patients [24, 25].
OAB and Long-Term Efficacy
Although the evidence of significant improvement in OAB symptoms with short-term use of PTNS, there is not yet a standardized protocol for maintenance therapy. Regimes varied between weekly and monthly stimulations depending on the patients’ and clinicians’ perception of symptoms control [26]. Two long-term follow-ups, The OrbiT and the Step trials, have showed that the majority of patients maintained a responder status, respectively, at 12 and 24 months with a mean interval of treatment of about 3 weeks. The withdrawal rate was 30–33 % [23, 27]. Considering these results, PTNS is an attractive alternative to drugs or implantable sacral nerve stimulation (SNS) for the long-term treatment. In contrast to SNS, patients can simply discontinue PTNS with no need to undergo surgery when become refractory to therapy. It’s been proposed a home-based transcutaneous tibial nerve stimulation (TTNS) as an attractive cheaper option for chronic treatment [28]. Despite that, the long-term use of PTNS therapy and its cost-effectiveness need to be examined further.
9.3.1.2 Neurogenic Bladder
Since the first study of McGuire, several trials have been published to better clarify the clinical and urodynamic effects of TNS in patients affected by neurogenic bladder [1, 29–35]. Finazzi Agrò et al. showed an improvement of urodynamic data in 9/14 patients affected by neurogenic detrusor overactivity (NDO) due to multiple sclerosis (MS), incomplete spinal cord lesion (SCL), and Parkinson disease (PD). Particularly in this paper by Finazzi Agrò et al, people with incomplete SCL showed to respond more than patients with central lesions [33]. De Seze et al. observed a significant improvement of LUTS and urodynamic filling parameters after daily 20 min sessions of TTNS for three months [35]. Gobbi et al. looked at the effect on QoL in patients suffering from MS. Eighty-nine percent of subjects reported a treatment satisfaction of 70 % with a significant improvement in most of QoL domains [31]. On the other hand, the use of PTNS is neurogenic voiding dysfunction is still more controversial.
9.3.1.3 Non-Obstructive Urinary Retention
Similarly to SNS, also PTNS has been proposed in patients affected by idiopathic or neurogenic non-obstructive urinary retention. The experience in this field of application is limited. On the basis of literature, the percentage of clinical success varied from 41 to 67 %. Vandoninck has reported an improvement of urodynamic parameters during voiding phase [36, 37].
9.3.1.4 Chronic Pelvic Pain
Only more recently, few studies have been published to assess the PTNS efficacy in treating chronic pelvic pain (CPP) syndrome. Based on literature, PTNS may be considered a treatment option in those “complicated” patients non-responder to standard conservative therapies. In particular it has been seen a reduction of VAS scale and an improvement of QoL questionnaire scores. Despite that, the percentage of responders in CPP patients seems to be lower (about 40–42 %) than that one reported in OAB patients [38, 39].
9.3.2 The Colorectal Experience
Extrapolation from SNS and urological evidence would suggest both sensory and motor neuromodulatory effects evaluated through anorectal physiology studies. These putative effects include upregulation of afferent rectal sensory perception and striated muscle function, allowing generation of increased maximum squeeze and resting pressure [40, 41]. There is also evidence of a reduction in spontaneous anal relaxations and rectal contractions [42–44]. Furthermore enhancement of rectal mucosal blood flow (as a surrogate marker of autonomic nervous function) has also been demonstrated as an alteration in the central neurotransmitter environment [45, 46].
Furthermore, it is well known from experimental data that somatic afferents from the skin are involved in neuromodulation of various autonomic functions. Afferent stimulation of the sciatic nerve inhibits gastrointestinal motility and that of the splanchnic nerves is responsible for mediating the inhibitory response [47].
In addition, with acupuncture needles placed either over the sacrum or perineal area, a stimulation regimen of 30 min per week for 10 weeks, followed by maintenance therapy after 1–3 months, a 50–85 % reduction in fecal incontinence episodes has been reported similar results to those after SNS and PTNS. The effects have sometimes been reflected even in the manovolumetric markers, with an increased tone in the internal sphincter, elevation of sensory thresholds, and an increased rectal volume capacity [48].
Upon these considerations, PTNS has been applied for several different coloproctological diseases.
One of the first experience was reported by Shafik et al. in 2003 who obtained an improvement in fecal incontinence scores in 78.2 % of the 32 cases treated [42]. Four years later Mentes et al. reported its application on partial spinal injury patients to be successful [49]. PTNS also showed its effectiveness in 28 patients included in the SUmit Trial who were also diagnosed for fecal incontinence: 45.5 % of PTNS patients improved, while only 18.2 % of sham subjects improved [27]. De la Portilla F in 2010 gave its contribution with a study conducted on 16 fecal incontinence patients: 10 of 16 improved in the short term, while only 5 of 16 maintained good results after 6-month period without treatment [50]. Govaert in a multicenter study reported improvement with >50 % decreased in incontinence episodes, in 59 % of patients treated at 1-year follow-up [51]. Boyle et al. obtained an important improvement in 68 % of 48 subjects treated with median number of incontinence episode per week decreased from 4 to 0 (p > 0.0001) [52]. The same year Findaly et al. evaluate the efficacy of PTNS on 13 patients with fecal incontinence of variable etiology: idiopathic, obstetric, and iatrogenic, and demonstrated subnormal physiology, but by contrast, all patients had intact sphincter complexes. Incontinence improved in all patients, with reduction in median episodes of wind, liquid, and solid to 0 episodes per month with 12 weeks treatment [53]. This was sustained for wind, but not liquid and solid, 1 month later. Although not presented in the initial paper, 10 of the 13 patients had a greater than 50 % reduction in incontinence. In common with Govaert et al., improvements were seen in those who had previously failed surgical intervention (sphincteroplasty and PTQ implants) [51, 53]. Another contribution derives from the experience of Hotouras et al., who published in 2012 his experience with 88 female incontinence patients showing a statistical improvement in the short term of Cleveland Clinic incontinence score, median deferment time, and median number of weekly incontinence episodes, and also that sphincter damage and altered rectal sensation did not appear to influence the outcomes. Hotouras enlarged his study to 100 patients affected by urge, passive, and mixed fecal incontinence: purely passive fecal incontinence did not show a significant improvement, while patients with urge FI (n = 25) and mixed FI (n = 60) demonstrated a statistically significant improvement in the mean CCF-FI score (11.0 ± 4.1 to 8.3 ± 4.8 and 12.8 ± 3.7 to 9.1 ± 4.4) with an associated improvement in the QoL score [54].
A pilot study from the St. Mark’s Hospital was realized in 2012 on 18 slow transit constipation patients: Wexner constipation score improved significantly as the PAC-QOL, stool frequency increased, and the use of laxatives decreased while there was no change in colonic transit time [55]. Again, Hotouras et al. (2012) published its approach to 20 unresponsive patients to PTNS over a complexive number of 100 patients treated: these patients were treated with sacral nerve stimulation (SNS), and 14 of them reported a significant therapeutic benefit with an improved incontinence score [56].
In conclusion the colorectal experience with PTNS showed encouraging results, mainly in the short term. The lower cost and invasiveness attribute to PTNS a possible future role in the flowchart treatment of fecal incontinence.
Enlarged cohort studies and high focused selected type of fecal incontinence patients need to clarify its effectiveness.
9.4 Conclusions
PTNS is nowadays a feasible option to treat pelvic floor organ dysfunctions as first- or second-line treatment. It is considered as a less invasive, safer alternative to SNS and more effective than transcutaneous stimulation. TTNS may have the advantage to be easily managed by patients at home.
No serious AEs have been reported in literature after PTNS. The majority of subjects, including children and frail older patients, seem to well tolerate the needle placement and the subsequent electrical stimulation.
Ongoing investigations improving our knowledge of neuromodulation mechanism of action are needed to increase the success rate.
New information about which factors may favor the time-duration efficacy, leading to a cost-effective treatment with impact on QoL, would afford specialists the opportunity to pursue a more appropriate individual treatment course.
Data from adequately powered comparative double-blind trials for PTNS treatment for both urinary and colorectal dysfunctions are necessary to be added to the existing evidence.
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree






