4. THE STOMACH
CONTROL
After studying this chapter you should be able to:
1. Understand the interplay of nervous and hormonal control of gastric function, and how this is coordinated by food in the gastrointestinal tract.
2. Understand how these control mechanisms result in coordinated function of the digestive system.
3. Understand how dysfunction can result in mucosal ulceration and how this can be diagnosed and treated.
4. Understand how dysfunction can result in secondary effects on the gastrointestinal tract and on systemic acid–base balance.
Introduction
Soluble substances in the food in the gastrointestinal tract and the mechanical pressure exerted by the food on the walls of the tract can stimulate or inhibit gastric secretion and motility. Nervous, paracrine and endocrine signals are involved. Peptic ulcer disease is a common condition in which gastric acid damages the mucosa of the duodenum or the stomach. In this chapter, the pathology and complications of this disease are used to emphasize the importance of the proper control of the functioning of the stomach (Case 4.1: 1).
Case 4.1
A 45-year-old man, from a family with a history of peptic ulcer disease, visited his general practitioner and complained of dull burning pain in his upper abdomen. This was associated with periodic nausea, vomiting, heartburn and loss of appetite. He was a heavy drinker and smoker. Upon examination, the pain was found to be localized to the epigastric region. The patient said that the pain was worse when his stomach was empty and was eased by eating a meal. He also complained of symptoms of hypersecretion of acid during the night. He often woke in the early hours with a burning sensation behind his lower sternum. He had been taking antacids that afforded him some relief. The doctor sent him for an endoscopy. This showed the presence of an ulcer in the proximal duodenum. He was advised to stop smoking and not to drink alcohol. He was initially prescribed ranitidine, an H2 blocker, the preferred treatment for ulcers at the time. However after 6 weeks, the symptoms from the ulcer had not resolved. He was then prescribed omeprazole, and symptomatic relief was quickly obtained. Unfortunately, the symptoms returned after approximately 8 months. Omeprazole treatment was started again, but this time it was prescribed in combination with a course of antibiotics. His symptoms disappeared, and by 2 years later he had suffered no further relapse.
After considering the details of this case you should be able to answer the following questions:
• Why did the general practitioner suspect from what the patient said that he might have a duodenal ulcer rather than a gastric ulcer?
• Which is the most common location for ulcers in the duodenum? Why do they usually occur at that location?
• Which is the most common site for ulcers in the stomach and why do they occur at that location?
• What is the mechanism of action of H2 receptor antagonists such as ranitidine? Why are these drugs usually effective in relieving the symptoms of peptic ulcer disease? Why should they be administered at night in this patient?
• What is the mechanism of action of omeprazole?
• Why was the patient given a course of antibiotics?
A leading role in the coordination of gastrointestinal functions is played by the hormone gastrin that is released from the stomach into the bloodstream during a meal. It stimulates both secretion and motility in the stomach. It also stimulates the blood supply and growth of the gastric mucosa. In addition, it controls many functions of other regions of the gastrointestinal tract and its associated glandular organs. Gastrin is released from G cells located mainly in the mucosa of the pyloric antrum, and so these cells are ideally placed to respond to the presence of ingested material in the stomach. Tumours of ectopic G cells, known as gastrinomas, can give rise to the Zollinger–Ellison syndrome. This rare disease is characterized by over-secretion of gastrin, which results in excessive secretion of acid, and hypermotility of the gastrointestinal tract. In this chapter the physiological and clinical importance of this hormone is illustrated by discussion of the functional abnormalities that arise in Zollinger–Ellison syndrome (Case 4.2: 1).
Case 4.2
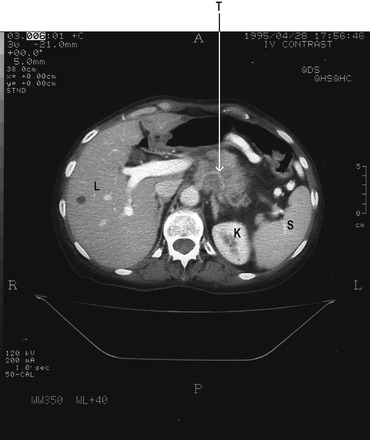
A 40-year-old woman who had been suffering, for several years, from intermittent abdominal pain and diarrhoea, visited her general practitioner. She had previously been diagnosed (by endoscopy) as having peptic ulcer disease, and had been prescribed omeprazole and a short course of antibiotics, but with no long-term relief of her symptoms. Consequently, she had undergone surgical division of the vagal nerves to the stomach (a selective vagotomy). Surprisingly, her symptoms persisted following the surgery. Further tests were initiated to investigate the possibility that they were due to a gastrinoma. Her basal acid secretion and her acid secretion in response to an injection of pentagastrin were investigated. This involved aspiration of gastric juice. A radioimmunoassay for gastrin was performed on a blood serum sample. She was restarted on a high dose of omeprazole to protect against further ulceration, and arrangements were made for her to have a further endoscopy. Hypertrophy of the gastric rugae, and ulceration extending into the second part of the duodenum, were seen. In the light of these observations, and the abnormal plasma gastrin level found, a computerized tomogram (CT scan) of her upper abdomen was performed. This demonstrated a mass in the pancreas (fig. 4.6). A laparotomy (abdominal operation) was performed and the surgeon confirmed that a tumour was present in the pancreas, and the tumour was removed. Subsequent histological analysis of the resected specimen demonstrated that the tumour was a gastrinoma. Removal of the tumour cured the patient’s symptoms and her serum gastrin concentration declined to within the normal range.
After studying the details of this case we can consider the following:
• What abnormalities in blood gastrin levels, gastric acid secretion, and pepsinogen secretion, might we expect to see in this patient? What changes might we expect following surgical vagotomy?
• Why was hypertrophy of the gastric mucosa present?
• Why was ulceration seen in the second part of the duodenum? Which other sites in the gastrointestinal tract are likely to be ulcerated in this condition?
• Which of the diagnostic tests used would have given indications that the condition was Zollinger–Ellison syndrome and not simple gastric or duodenal ulceration?
• What is the rationale for treating this condition with a high dose of omeprazole?
• What are the explanations for the patient’s diarrhoea?
• What are the physiological consequences of excessive gastrin and acid production?
Control of gastric secretion
The control of secretion of gastric juice involves extrinsic and intrinsic nerves, hormones and paracrine mediators.
Hormonal control
Gastrin
Gastrin is a hormone that is secreted from the G cells in the stomach. It stimulates gastric juice secretion, and has a general role in the preparation of the gastrointestinal tract for the digestion and absorption of food. It was the first hormone to be discovered (Box 4.1).
Box 4.1
Gastrin was the first hormone to be discovered. The existence of a substance that is released into the blood in response to food in the stomach, and which circulates to stimulate acid secretion, was first proposed by Edkins in 1905. However, when it was realized that histamine, a substance present in abundance in gastric mucosa, stimulated acid secretion, it was assumed that this was the main mediator. It was not until 30 years later that Grossman and his colleagues demonstrated the existence of a blood-borne factor that stimulated acid secretion. They isolated a pouch from the body of the stomach in a dog and transplanted it into the neck region. They showed that food placed in the antrum, which remained in situ, stimulated acid secretion in the transplanted pouch. Increased secretion occurred even if the antrum was denervated, indicating that the stimulus was a blood-borne factor released from the gastric antrum; that is, a ‘hormone’. In 1964, Gregory and Tracey isolated the pure peptide hormone from hog stomach. It was subsequently called gastrin.
Biologically active forms of gastrin
In the normal human, gastrin is produced mainly in the gastric antrum, although small amounts are produced in the proximal small intestine. Two major forms of gastrin exist, gastrin-34 (G34, composed of 34 amino acids) and gastrin-17 (G17, composed of 17 amino acids). In humans, over 90% of the gastrin present in the antral mucosa is the G17 form. Gastrin-17 has a half-life in the circulation of approximately 6min and G34 a half-life of approximately 36min. Both peptides stimulate gastric acid secretion. The short half-life of the G17 form is consistent with its main influence being via local receptors in the stomach. The active part of the molecule is the carboxy-terminal tetrapeptide sequence.
The active sequence is contained in the pentapeptide drug pentagastrin, a synthetic drug that consists of the C-terminal tetrapeptide to which a substituted β-alanine has been added to stabilize the molecule. It exhibits all the physiological actions of gastrin. Clinically, it is administered as an alternative to histamine (see Ch. 3) to test gastric secretion.
G cells and gastrin secretion
In normal individuals, most of the G cells, which secrete gastrin, are found in the mucosa of the gastric antrum, although some (<20%) are present in the duodenal mucosa. The G cells comprise less than 1% of the mucosal cells. In the human, these cells, together with other endocrine cells, are found between the basal and neck regions of the gastric glands (see Ch. 3). The mature cells are replaced from immature precursor cells located in the isthmus of the antral glands. The turnover of the G cells is slow (unlike that of the epithelial cells). It is stimulated by gastrin. G cells (Fig. 4.1) are ‘open’ APUD endocrine cells (see Ch. 1). In these cells, microvilli are present along their apical surfaces, which are in contact with the lumen of the stomach. This structural feature of the cell enables it to sample the gastric contents. Receptors present on the luminal surface membrane sense chemical substances in food, known collectively as ‘secretogogues’, which regulate the release of gastrin (see below). Gastrin is stored in secretory granules present along the basolateral border of the cell which lies in close proximity to the blood vessels. It is released into the circulation at the basolateral membrane in response to neural, endocrine or paracrine stimuli, and by local factors in the lumen of the stomach.
Gastrin receptors
A variety of cell types possess specific surface receptors for gastrin. The oxyntic cell is the type that has been most studied. Interestingly, gastrin and cholecystokinin (CCK, a hormone secreted by the duodenal mucosa) have the same active carboxy-terminal tetrapeptide and act on the same receptors. There are two such receptors, the CCK-A receptor, present in the pancreas and the gall bladder, and the gastrin-CCK-B receptor, present on the ECL cell and the oxyntic cell. The two hormones exhibit different potencies at these receptors. CCK has a 10-fold higher potency than gastrin at the CCK-A receptor, and gastrin is the more potent at the CCK-B receptor. This difference in binding affinities between gastrin and CCK at the two CCK receptors is the main reason for their different patterns of biological activity. CCK exerts its main physiological effects on the biliary tree and the pancreas where CCK-A receptors predominate, whereas gastrin is more potent in the stomach.
Cellular actions of gastrin on acid secretion
Oxyntic cell
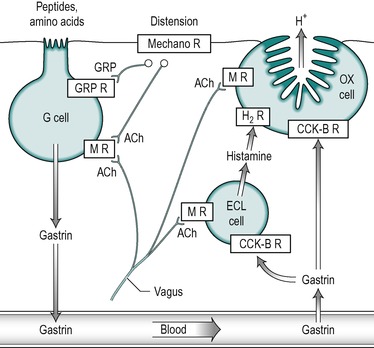
In the healthy individual the secretion of acid and intrinsic factor by the oxyntic cell are regulated in parallel, so that stimulation of acid secretion is accompanied by increased secretion of intrinsic factor. Gastrin stimulates acid secretion by two mechanisms: it stimulates the oxyntic cell directly, and it stimulates it indirectly through stimulation of the ECL cell to release histamine which in turn stimulates the oxyntic cell (Fig. 4.2). It binds to CCK-B receptors on the cell membranes in both types of cell. The result of this stimulation is the incorporation of proton pumps into the canalicular membrane of the oxyntic cell (see Ch. 3). Gastrin also stimulates the expression of the gene for the proton pump in the oxyntic cell, thereby increasing its synthesis.
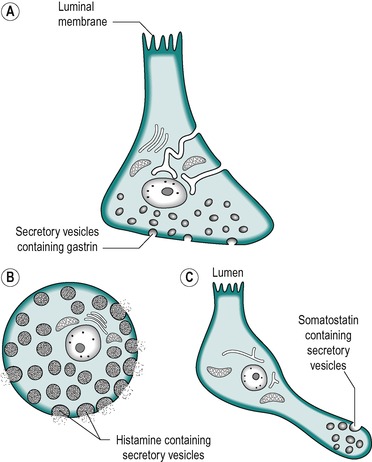 |
| Fig. 4.2 (A) Gastrin-secreting (G) cell. (B) Histamine secreting (ECL) cell. (C) Somatostatin secreting cell. |
Gastrin also has trophic actions. It controls the growth and proliferation of a variety of cell types in the gastric mucosa, including ECL cells and the precursors of oxyntic cells. Hyperplasia of ECL cells and oxyntic cells occurs in conditions where hypergastrinaemia is present (see Case 4.2: 2).
ECL cell and histamine
Gastrin binds with a high affinity to CCK-B receptors on ECL cells, to cause the release of histamine (Fig. 4.2). Histamine acts in a paracrine manner on the oxyntic cell to release acid. The binding of gastrin to the ECL cell also stimulates histamine synthesis from histidine (see below).
It is produced in large amounts by the gastric mucosa. It is produced by decarboxylation of histidine:

Histamine acts on H2 receptors on the oxyntic cells to release acid (Fig. 4.2) via a cyclic AMP-mediated mechanism. Its presence is necessary for the secretion of normal amounts of acid. Thus inhibition of the enzyme histidine decarboxylase reduces acid secretion.
Neural control of gastric secretion
The functions of the stomach are controlled by intrinsic nerves in the internal nerve plexi of the enteric nervous system and by extrinsic nerve fibres in the vagus nerve and sympathetic nerves (see Ch. 1). Axons of nerve fibres (in the intramural plexi) innervate both secretory cells and smooth muscle cells. In general activation of cholinergic fibres stimulates gastric secretion and motility. Cases 4.1 and 4.2: 2 shows the arrangement of the vagal cholinergic nerve trunks that innervate the stomach. Activation of adrenergic fibres generally inhibits secretion and motility.
It should also be noted that a number of sensory nerves leave the stomach and travel in the vagus nerve and the sympathetic nerves. Sensory nerves in the stomach also provide afferent paths of intrinsic reflex arcs, which travel in the intramural plexi of the stomach. This provides some intrinsic control of smooth muscle contractions and gastric juice secretion.
Acetylcholine and gastrin-releasing peptide
Acetylcholine released from cholinergic nerve fibres in local nerves can stimulate oxyntic cells to release acid, or G cells to secrete gastrin. Some fibres in the vagus nerve also contain gastrin-releasing peptide (GRP), which exists as two major forms, in which the active site is a nonapeptide. The structurally similar peptide bombesin that has been extracted from the skin of the frog Bombina bombina has similar actions. GRP released from nerves in the stomach stimulates gastrin release from the G cells (Fig. 4.2). It probably also stimulates acid release by a gastrin-independent mechanism. This interaction of the neural and gastrin control mechanisms facilitates a rapid response to food ingestion.
Inhibitory control of acid secretion
Feedback control via acid
Gastric acid secretion is blocked if the contents of the stomach become too acid (pH 3.0, or lower). This is a negative feedback mechanism that prevents the gastric contents (and probably more importantly the duodenal contents) from becoming too acid. When the acidity of the stomach reaches pH 2.0 it is virtually impossible to stimulate gastrin release by any means. The inhibition is indirect and is exerted via inhibition of gastrin release. In conditions where achlorhydria (lack of acid secretion) is present (such as some types of pernicious anaemia), there are usually high levels of gastrin in the blood because this feedback mechanism cannot operate. The inhibitory action of acid on gastrin secretion is due to its action to stimulate the release of the hormone somatostatin from D cells in the mucosa. Somatostatin is a potent inhibitor of acid secretion; it inhibits gastrin secretion from G cells and histamine secretion from ECL cells. Furthermore, the D cells exhibit gastrin-binding sites, and gastrin itself can stimulate somatostatin release from these cells. However these binding sites are probably CCK-A receptors which are more sensitive to CCK than to gastrin. Stimulation of somatostatin release via these receptors is therefore probably normally due mainly to circulating CCK (see below).
In both peptic ulcer disease and in Zollinger–Ellison syndrome, acid secretion is increased but gastrin levels are only increased in Zollinger-Ellison syndrome (Case 4.1 and Case 4.2).
Case 4.1
Causes and diagnosis
Individuals with duodenal ulcer disease often have a family history of the condition. The duodenum is the most frequent site for ulcer formation, the duodenal cap being the most vulnerable area. Excessive secretion of acid and pepsinogen are directly implicated in chronic ulceration of the duodenum. High H+ concentration can lead to the breakdown of the protective mechanisms of the mucosal barrier (see Box 4.3). Patients with simple duodenal ulcer usually have a high basal acid output with normal levels of serum gastrin. In contrast, patients with gastric ulcer appear to have normal or slightly low acid secretion (Table 4.1). In gastric ulcer the primary defect may be a reduced ability of the mucosa to withstand damage by acid and pepsin (see Case 4.1 and Cases 4.1 and 4.2).
| Typical mean values are given for acid secretion and blood gastrin levels in normal subjects, and patients with simple gastric ulcer, duodenal ulcer or Zollinger–Ellison (ZES) syndrome. Maximum acid output is elicited by injection of pentagastrin (6μg/kg). | ||||
| Acid | Rate of acid secretion | Blood gastrin (pmol/L) | ||
|---|---|---|---|---|
| Basal day (mmol/h) | Basal night (mmol/12h) | Maximum (mmol/h) | ||
| Normal | 1–5 | 18 | 25 | 30 |
| Gastric ulcer | 1–5 | 8 | 25 | 30 |
| Duodenal ulcer | 4–10 | 60 | 40 | 30 |
| ZES | 45 | 120 | 55 | 650 |
An endoscopy can be carried out on patients with suspected peptic ulcer to locate an ulcer and confirm that it is not a tumour (which can present with similar symptoms).
The patient described in Case 4.1 demonstrated hypersecretion of acid which is more typical of a duodenal ulcer than a gastric ulcer. He also complained of heartburn and pain when his stomach was empty. Eating provided relief because food buffers the acid. Symptoms of hypersecretion at night are supposedly more usually seen with a duodenal rather than a gastric ulcer but in practice there is often overlap of symptoms between the two types. In the case of a duodenal ulcer the symptoms usually last for a few weeks followed by a remission.
Gastric ulcer
Although a family history is often present in duodenal ulcer inheritance appears to be unimportant in gastric ulcer. In a patient with gastric ulcer the pain is poorly localized but may be perceived in the midline area. It occurs at any time but is often worse during or after a meal. Physical examination does not usually demonstrate epigastric tenderness. There is not usually nausea or vomiting and food does not ease the pain. In practice, however, the differential diagnosis between gastric and duodenal ulcer cannot be ascertained on the basis of symptoms alone.
Figure 4.3 shows an X-ray of the stomach of a patient with a chronic gastric ulcer.
An endoscopy would demonstrate a gastric ulcer. In the case of a suspected gastric ulcer it is important to ask whether the patient has lost weight and to take a biopsy of the ulcerated mucosa because there is a risk of malignancy, which is not seen in the case of a duodenal ulcer.
Case 4.2
Causes and diagnosis
In 40% of patients with Zollinger–Ellison syndrome familial inheritance has been recorded. The syndrome can be inherited in an autosomal dominant fashion, so relatives may be affected. For this reason it is important to obtain a family history from patients diagnosed with the disease.
The gastrinomas present may be ectopic, commonly presenting in the pancreas. They may be small and difficult to locate. In 60% of patients, the tumours are malignant. The gastrinoma tumours secrete excessive amounts of gastrin into the portal blood stream. The high serum gastrin levels elicit massive secretion of acid from the oxyntic cells. It is the basal acid secretion (which occurs between meals) that is stimulated to the greatest extent by the high gastrin levels. The secretion of gastrin from the gastrinomas (as with many other secretory tumours) is independent of secretogogues such as peptides in the stomach. Thus secretion of acid during a meal is not abnormally affected. Table 4.1 shows typical values for the basal and maximum acid secretion (induced by injection of pentagastrin), and serum gastrin levels in normal individuals, and in patients with gastric ulcer, duodenal ulcer, or Zollinger–Ellison syndrome. The basal acid output is usually considerably elevated in Zollinger–Ellison syndrome. However, the maximum acid output measured after pentagastrin injection is not increased proportionately, unlike in normal individuals or patients with peptic ulcer disease. The basal acid output is usually not less than 60% of the maximum output, and is often the same as the maximum output.
In normal individuals, a low pH in the stomach lumen inhibits acid secretion by inhibiting gastrin secretion from the antral G cells (see below) but secretion by gastrinomas is independent of this feedback control.
Pepsinogen secretion is also stimulated by gastrin, so its secretion is also usually abnormally high in Zollinger–Ellison syndrome.
Diagnosis
The diagnosis of Zollinger–Ellison syndrome requires consideration of the results of a number of different procedures. Basal acid secretion is dramatically elevated, and serum gastrin levels are often elevated over 10-fold, as a consequence of the uncontrolled secretion (Table 4.1). However, high basal acid production by the stomach and high levels of gastrin in the blood are merely suggestive of the condition.
The ‘secretin test’ has been used in the past to assist the diagnosis of Zollinger–Ellison syndrome, but now that gastrin levels can be measured directly and accurately by radioimmunoassay, it is no longer often employed. The basis of the test depends on the fact that whereas secretin infusion normally inhibits acid secretion during the intestinal phase of digestion (by acting on the oxyntic cell directly and on the G cell), and normally has little effect on basal acid secretion between meals, it stimulates secretion of gastrin from ectopic gastrinomas; the so-called ‘paradoxical’ effect of secretin in Zollinger–Ellison syndrome.
Radiological imaging (Fig. 4.3), particularly magnetic resonance imaging (MRI) can detect lesions as small as 1cm in diameter. When malignant lesions are present, metastases are usually visible in the liver. Exploratory surgery is required to confirm the lesion (and to remove benign tumours). Malignant tumours are usually treated with proton pump inhibitors to simply control the symptoms of excessive acid secretion.
Note: Gastrin has trophic actions on the mucosa of the gastrointestinal tract. It is a growth factor for the stomach mucosa and in Zollinger–Ellison syndrome the high levels can stimulate hypertrophy of the mucosa. The rugal folds may become extremely thick. This can sometimes be seen in medical imaging procedures such as the barium meal test.
Somatostatin
Somatostatin exists predominantly as the 14-amino-acid peptide somatostatin-14 in D-cells in the fundic and antral mucosa. It is released from cytoplasmic processes on the D cells in the antrum in the vicinity of its target cell, the G-cell (Fig. 4.4). It binds to somatostatin-2 (ST-2) receptors on the G cells. It acts primarily in a paracrine manner via diffusion in the intercellular spaces, but it also acts systemically through its release into the local mucosal circulation. Somatostatin also acts on ECL cells to inhibit histamine release. These interactions are outlined for the antrum region in Figure 4.4. Somatostatin also acts upon the oxyntic cells in the fundus to inhibit the release of acid directly.
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree