, Carmen L. Menendez2, Rodolfo Montironi3 and Liang Cheng4
(1)
Department of Surgery and Pathology, University of Cordoba Faculty of Medicine, Cordoba, Spain
(2)
Pathology Department, Hospital de Cabueñes, Gijón, Spain
(3)
Department of Biomedical Sciences and Public Health, Polytechnic University of the Marche Region (Ancona), Torrette, Italy
(4)
Department of Pathology, Indiana University School of Medicine, Indianapolis, Indiana, USA
3.1 Basic Anatomy and Histology
3.1.1 The Prostate
The adult prostate surrounds the urethra and is located posterior to the inferior symphysis pubis, superior to the urogenital diaphragm, and anterior to the rectum. It measure 5 cm × 4 cm × 3 cm and weighs 20 g from ages 20 to 50, with an increase to 30 g from ages 60 to 80.
The three-zone model defines the central zone, the transition zone, and the peripheral zone.
Cancer tends to arise in the peripheral zone (20 % do arise in the transition zone), and BPH typically arise in the transition zone (Figs. 3.1, 3.2, 3.3, 3.4, 3.5 and 3.6). The central zone is more resistant to disease. The prostatic urethra exits the prostate at the apex where it is continuous con the membranous urethra.

Fig. 3.1
Simple retropubic prostatectomy showing benign hyperplasia of the transition zone
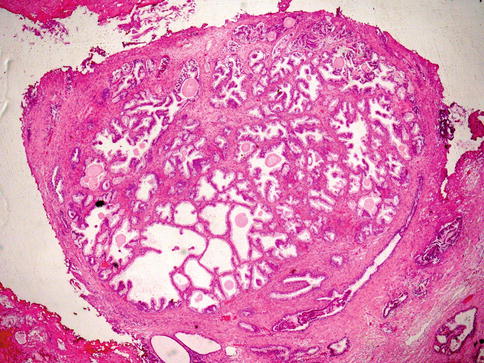
Fig. 3.2
Low power histology of a nodule of benign hyperplasia of the prostate
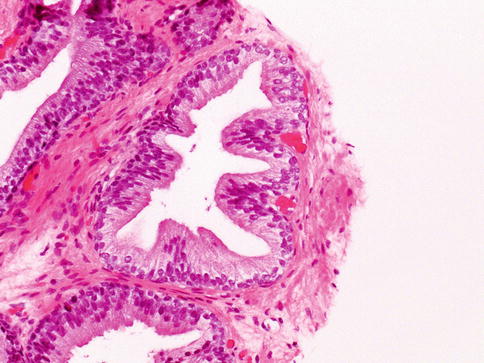
Fig. 3.3
Microscopically, prostate glands show basal cell layer and the secretory cells towards the lumen
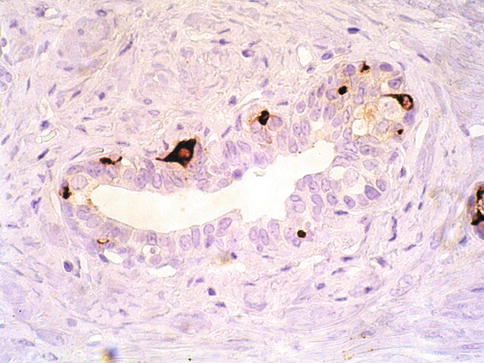
Fig. 3.4
Neuroendocrine cells are present within the basal cells but may extend to luminal surface
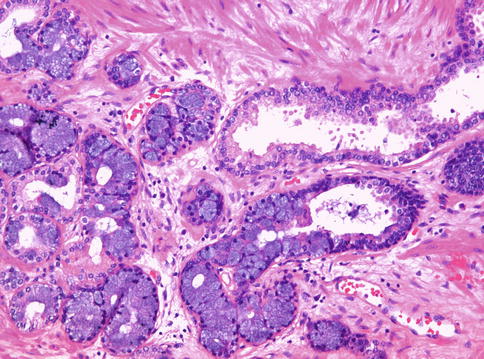
Fig. 3.5
Mucinous metaplasia may be occasionally seen in some prostate glands
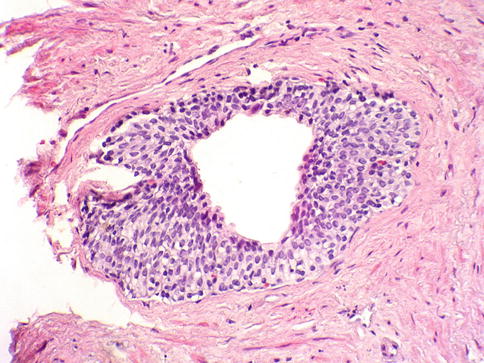
Fig. 3.6
Urothelial metaplasia is focally seen in some prostate glands
The paired ejaculatory ducts run through the central zone from the seminal vesicles to their exit at the posterior urethral protuberance, known as the verumontanum (Fig. 3.7). Within the verumontanum is the prostatic utricle, located between the ejaculatory ducts (Fig. 3.8).
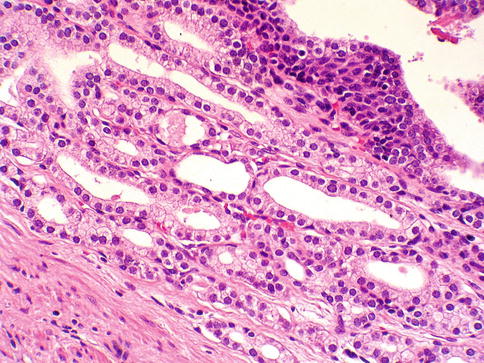
Fig. 3.7
Histology of the verumontanum glands
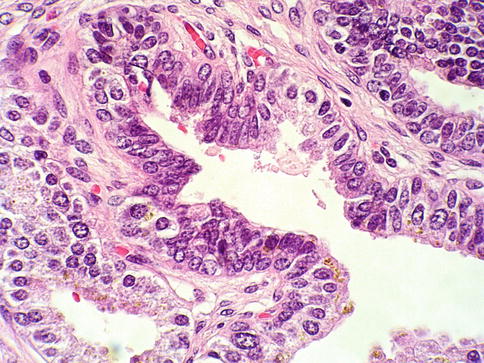
Fig. 3.8
Microscopic appearance of ejaculatory ducts
The anterior fibromuscular stroma is present anteriorly over the prostate and extends from the bladder neck to the apex of the prostate.
Adult prostate gland is a branching duct-acinar embedded in a fibromuscular stroma. The epithelium has two cell layers: the luminal/secretory and the basal layer with some neuroendocrine cells in the epithelium (Figs. 3.3 and 3.4). The cytoplasm of secretory cell is cleared with yellow-brown pigment (lipofuscin) but not mucin.
Secretory cells are positive for CKAE1/AE3, CK 8–18, PSA, PAP, PSMA, p501S and NKX3-1. P504S (racemase) may show a focal non-circumferential positivity in secretory cells.
Lipofuscin can also be found in the cytoplasm of seminal vesicle and ejaculatory duct epithelium (Fig. 3.9).
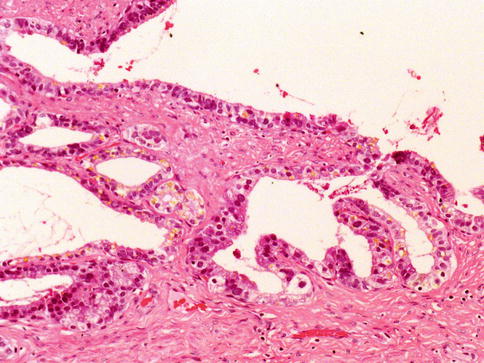
Fig. 3.9
Lipofucsin in seminal vesicle epithelium
Basal cells have a dense cytoplasm, and small hyperchromatic nuclei. They react with p63, CK5/6 and high molecular weight CK clone 34βE12 (CK903).
Neuroendocrine cells are less frequent and show an immunohistochemical profile more similar to secretory cells with variable androgen receptor and PSA/PAP expression. They express a number of immunohistochemical markers including chromogranin, synaptophisin, neuron-specific enolase, and many others.
Urothelium is also normally present in the prostate sometimes showing a cleared cytoplasm. It is PSA negative and p63 positive.
Intraluminal contents of normal prostate glands include degenerated epithelial cells, corpora amylacea and calculi. Rarely, it may contain focal blue-tinged mucin, pink amorphous acellular secretions, and crystalloids.
The prostatic stroma, including fibroblasts, smooth muscle, vasculature and nerves, and rarely adipocytes (carcinoma in fat should be viewed as extraprostatic extension). There are zonal differences in prostatic fibromuscular stroma density.
The prostate does not have a true capsule. The so called outer prostatic “capsule” is a band of concentrically placed fibromuscular and vascular tissue that is inseparable from prostatic stroma and surrounding fascia. This is absent in the apex.
Histologically, nerves are seen in the periprostatic neurovascular bundle, in the outer fibromuscular band, and in the prostate itself. Paraganglia (cleared cells that should not be mistaken as carcinoma) are usually adjacent to neurovascular bundles but may be seen deeper. Posterolateral prostate shows abundant adipose tissue together with neurovascular bundles.
Bulbourethral Cowper glands are extrinsic to the prostate but eventually may be seen in prostate biopsies. Histologically they are tubuloalveolar glands with lobules of acini, admixed with excretory ducts and ductuli. Frequently they are associated with skeletal muscle fibers. The cells are cuboidal to columnar, pale-staining mucinous cytoplasm and small, bland, basally located nuclei. A basal cell layer is not readily apparent with H&E. Immunohistochemistry remains unsettled with studies suggesting PSA or PSAP positive or negative. It is unclear if 34βE12 highlights basal cells but acinar cells are 34βE12 negative.
Distorted colorectal epithelium can potentially be confused with prostatic adenocarcinoma since it is negative for basal cell markers 34βE12 and p63.
3.1.2 The Seminal Vesicles and Ejaculatory Ducts
Seminal vesicles lie posterolateral to the base of the urinary bladder and anterior to Denonvilliers fascia. Seminal vesicle wall consists of a thick circumferential coat of smooth muscle and the ejaculatory ducts are surrounded by a collagenous stroma. Uncommonly, one may see small hyaline globules in the SVs wall (Fig. 3.10).
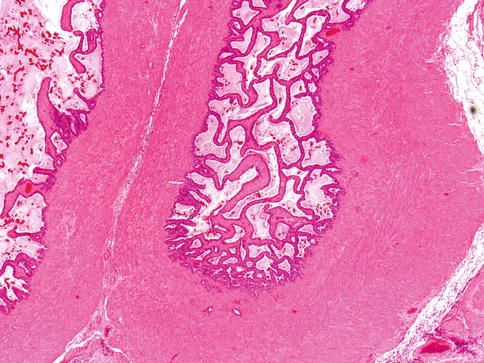
Fig. 3.10
Seminal vesicle wall consists of a thick circumferential coat of smooth muscle
Seminal vesicle and ejaculatory ducts share histogenesis from the wolffian system with the prostatic central zone.
The SVs epithelium is composed of ducts and acini with a two-cell lining layer of secretory cells and basal cells. Lipofuscin (yellow-brown pigment) can be found in the cytoplasm of secretory cells in SVs and ejaculatory duct epithelium, and rarely in stromal cells. It is characteristic to see scattered nuclei exhibiting enlargement and degenerative-type hyperchromasia. This is a helpful feature to separate SVs/ejaculatory ducts epithelium from prostate epithelium.
The immunoprofile of secretory cells of SVs/ejaculatory ducts is CKAE1/AE3+, CAM5.2+, PAX-2+, MUC 6+, PSA-, PAP- and P504S (racemase) -. Basal cells are p63+.
3.2 Adenocarcinoma of the Prostate
The incidence of prostate cancer (PCa) has raised dramatically in the past 25 years owing to early detection programs that employ serum prostate-specific antigen (PSA). In developed countries, PCa is the most commonly diagnosed non-skin malignancy in men.
Multiple factors contribute to the development of PCa as well as to its progression to an androgen-independent state: dietary factors, inherited susceptibility factors, gene defects, and androgens and their receptors.
Most prostate cancer patients are asymptomatic nowadays, detected by digital rectal examination and imaging methods in the setting of high serum PSA level. Most cancers arise in the peripheral zone, so that transition zone enlargement sufficient to cause bladder outlet obstruction usually indicates hyperplasia.
The test most used is serum PSA and its derivatives. PSA is elevated beyond the arbitrary cut-off point of 4.0 ng/ml in the majority of patients with prostate cancer (sensitivity of 20 % and specificity of 60–70 %). It may also be greater than 4.0 ng/ml in benign conditions including benign prostatic hyperplasia (BPH).
After therapy of localized PCa, PSA is expected to decline to a nadir of <0.2 ng/mL. PSA elevation afterward (≥0.2 ng/mL) on two occasions is considered biochemical recurrence.
3.2.1 Preneoplastic Lesions and Conditions
3.2.1.1 Prostatic Intraepithelial Neoplasia
Prostatic intraepithelial neoplasia (PIN) refers to the preinvasive end of the continuum of cellular proliferations within the lining of prostatic ducts, ductuli, and acini (Figs. 3.11, 3.12, 3.13 and 3.14).
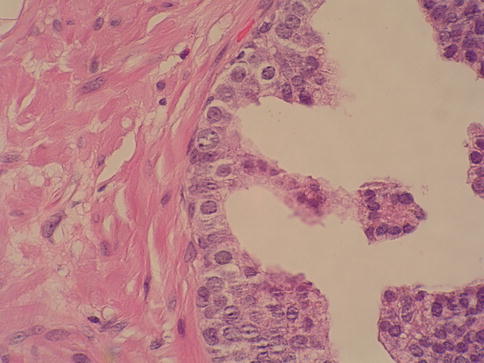
Fig. 3.11
Microscopic aspect of prostate intraepithelial neoplasia
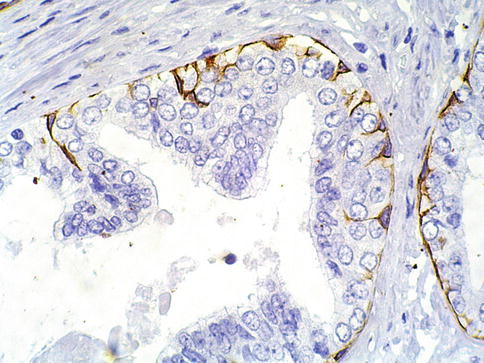
Fig. 3.12
Discontinuous basal cell layer seen with basal cell cytokeratin in prostate intraepithelial neoplasia
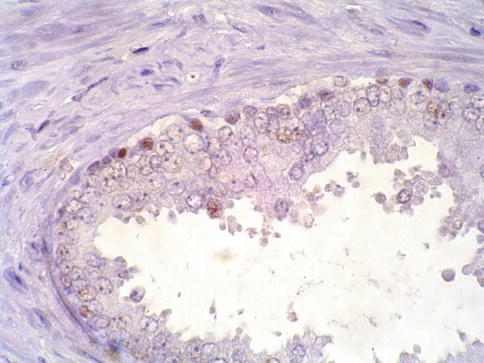
Fig. 3.13
Discontinuous basal cell layer seen with p63 in prostate intraepithelial neoplasia
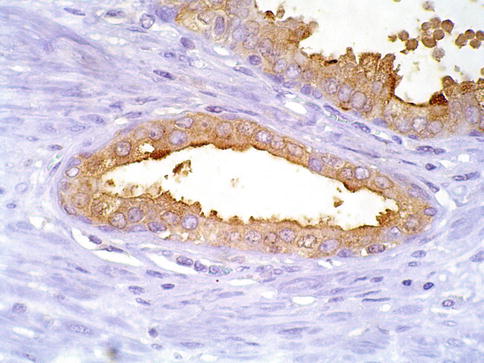
Fig. 3.14
PSA cytoplasmic expression in prostate intraepithelial neoplasia
It is currently recommended compression of the PIN classification into two grades: low-grade (formerly PIN grade l) or high-grade PIN (formerly PIN grades 2 and 3) (HGPIN) (Table 3.1).
Table 3.1
Differential diagnoses of prostate adenocarcinoma
Common:
Atrophy
Post-atrophic hyperplasia
Partial atrophy
Basal cell hyperplasia
Atypical adenomatous hyperplasia (adenosis)
Inflammatory-associated atypia
High-grade PIN
Less common:
Cowper’s gland
Nephrogenic metaplasia
Clear cell cribriform hyperplasia
Seminal vesicle/ejaculatory ducts
Paraganglia
Xanthoma
3.2.1.2 Diagnostic Criteria for HGPIN
The classification of PIN into low-grade and high-grade is chiefly based on the cytological characteristics of the cells. The nuclei of cells composing low-grade PIN are enlarged, vary in size, have slightly increased chromatin content, and possess small or inconspicuous nucleoli.
HGPIN is characterised by cells with large nuclei of relatively uniform size, an increased chromatin content, which may be irregularly distributed, and prominent nucleoli that are similar to those of carcinoma cells. The basal cell layer is intact or rarely interrupted in low-grade PIN, but may have frequent disruptions in high-grade lesions.
There are four main patterns of HGPIN: tufting, micropapillary, cribriform, and flat. The tufting pattern is the most common, present in 97 % of cases, although most cases have admixed patterns. There are no known clinically important differences between the architectural patterns of HGPIN, and their recognition appears to be only of diagnostic utility. Other unusual patterns of HGPIN include the signet ring-cell pattern, small cell neuroendocrine pattern, mucinous pattern, inverted-type, squamous-type and foamy pattern (Figs. 3.15, 3.16, 3.17, 3.18, 3.19, 3.20, 3.21 and 3.22).
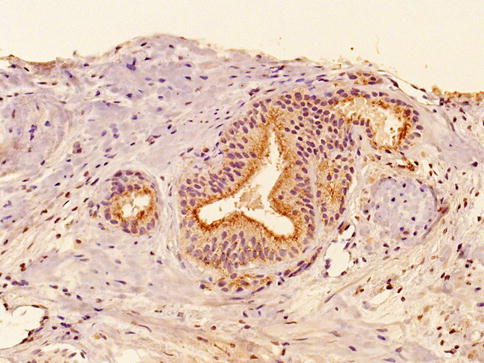
Fig. 3.15
Racemase cytoplasmic expression in prostate intraepithelial neoplasia
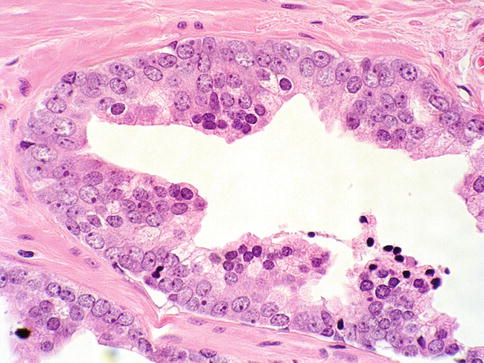
Fig. 3.16
Prostate intraepithelial neoplasia with tufting histologic pattern
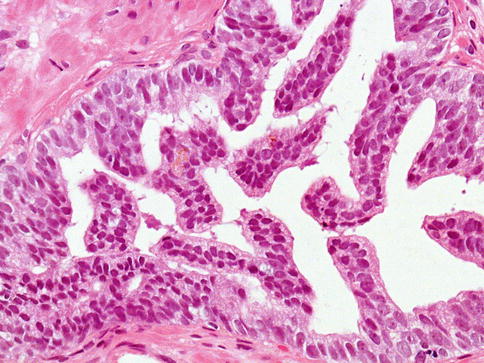
Fig. 3.17
Prostate intraepithelial neoplasia with micropapillary histologic pattern
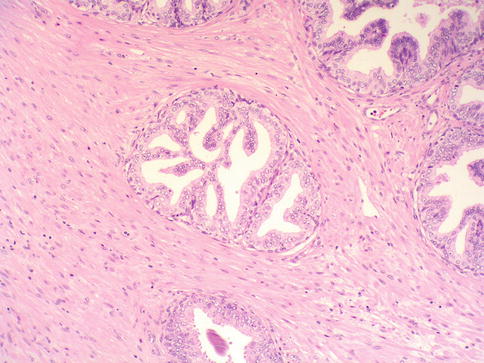
Fig. 3.18
Prostate intraepithelial neoplasia with cribriform histologic pattern
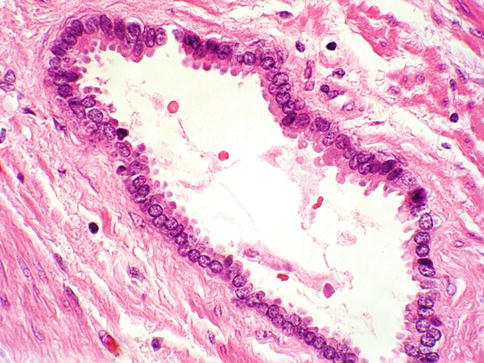
Fig. 3.19
Prostate intraepithelial neoplasia with flat histologic pattern
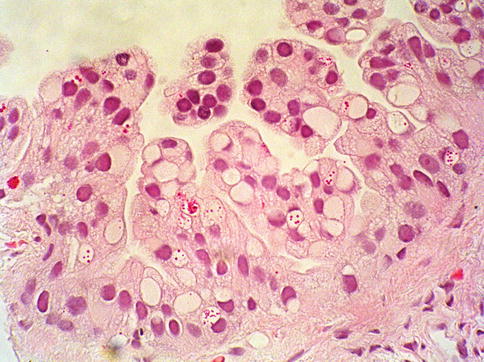
Fig. 3.20
Prostate intraepithelial neoplasia with cytoplasmic vacuoles (signet-ring) histologic pattern
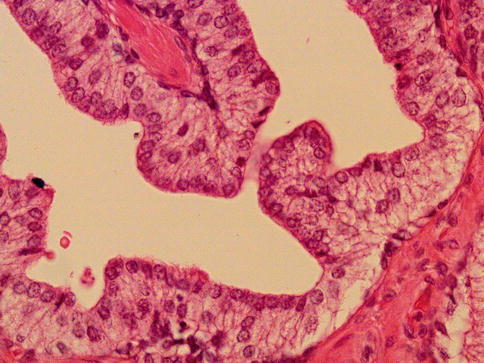
Fig. 3.21
Prostate intraepithelial neoplasia with inverted histologic pattern
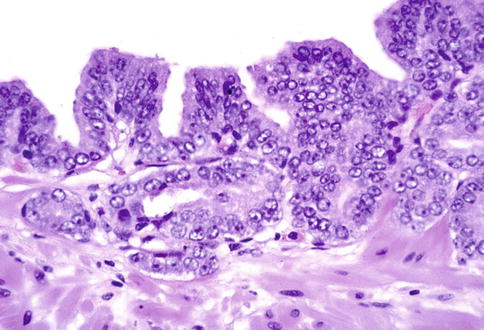
Fig. 3.22
Prostate intraepithelial neoplasia with microinvasive histologic pattern
HGPIN is multicentric in 70 % of radical prostatectomies with cancer, including 65 % of those involving the non-transition zone and 7 % of those involving the transition zone; 2 % of cases have concomitant single foci in all zones.
The peripheral zone of the prostate, the area in which the majority of prostatic carcinomas occur (70 %), is also the most common location for HGPIN where frequently is multicentric.
Early stromal invasion (PIN with microinvasion), the earliest evidence of carcinoma, occurs at sites of acinar out pouching and basal cell disruption in acini with HGPIN. Such microinvasion is present in about 2 % of high power microscopic fields of HGPIN, and is seen with equal frequency with all architectural patterns.
Evidence linking HGPIN and prostate cancer has been found in morphological, immunohistochemical, morphometric, molecular and genetic studies. Virtually all such studies have indicated that HGPIN is related more closely to prostate cancer than to benign epithelium. HGPIN was more extensive in small cancers than in larger cancers, presumably due to “overgrowth” or obliteration of HGPIN by larger cancers.
Discrepancies in the diagnosis of HGPIN were greatest between HGPIN with cribriform proliferations.
When the small atypical acini are too numerous and too crowded to be outpouchings or simply tangential sections, then cancer can be diagnosed. Immunohistochemical stains for the basal cell markers are of value in selected cases.
HGPIN shows a discontinuous basal cell layer when labelled with the antibody to high-molecular-weight keratin or p63. HGPIN is considered nowadays the most frequent cause of misdiagnosis of PCa in needle prostate biopsies.
HGPIN has a limited predictive value as a marker of adenocarcinoma in subsequent biopsy when single and unilateral. Bilateral and multifocal HGPIN increases likelihood of PCa being diagnosed in up to 10 %.
Cancer detection rate in patients with low-grade PIN is identical to that in patients who underwent repeat biopsy for persistent elevated serum PSA or because of an abnormal digital rectal examination.
3.2.1.3 Atypical Adenomatous Hyperplasia
Atypical adenomatous hyperplasia (AAH, adenosis) is characterized by a circumscribed proliferation of closely packed small glands that, rather than appearing invasive, tends to merge with the surrounding, histologically benign glands.
AAH frequently demonstrates budding acini from adjacent foci of benign hyperplastic glands, and the cells have clear cytoplasm with variable intraluminal secretions. Architecturally, AAH resembles well-differentiated adenocarcinoma of low Gleason score, and most cases of Gleason primary pattern 1 cancer are now considered within the spectrum of AAH. Recognition of the basal cell layer excludes the diagnosis of carcinoma.
Unfortunately, identification of the basal epithelium is often difficult, as it is usually attenuated and may be discontinuous in AAH, and this may be the rule in needle biopsy specimens.
Recognition of the basal cell layer may be facilitated by use of an antibody to p63 or to high-molecular-weight cytokeratin. An additional compelling feature is the observed positive immunoreactivity of AAH foci with Racemase (P504S). AAH lacks TMPRSS2-ERG Gene fusion as recently demonstrated.
Nuclear and nucleolar morphology is the key differentiating feature between AAH and carcinoma, but this has less power than the presence of basal cells after immunohistochemistry. About 85 % of cases of AAH are located in the transition zone. The incidence of AAH in prostate specimens is largely variable in reported series (2–23 %) (Figs. 3.23, 3.24, 3.25 and 3.26).
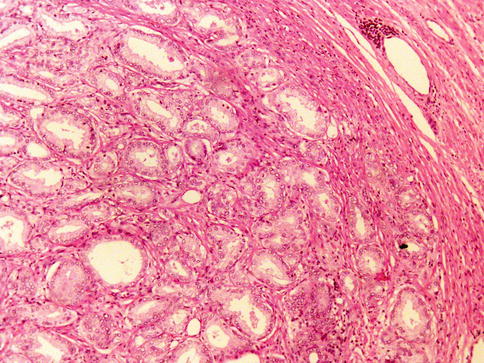
Fig. 3.23
Microscopic features of atypical adenomatous hyperplasia (adenosis) of the prostate (low power view)
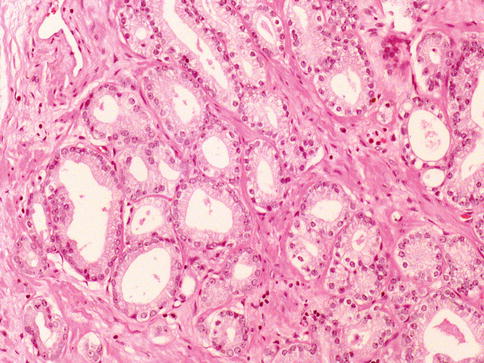
Fig. 3.24
Microscopic features of atypical adenomatous hyperplasia (adenosis) of the prostate (high power view)
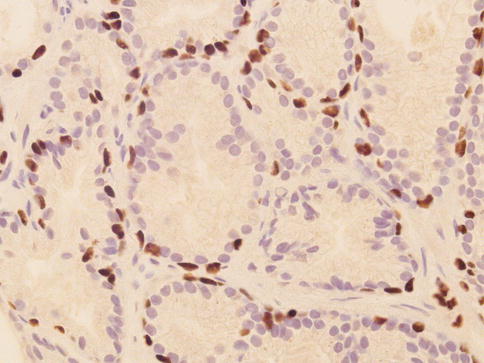
Fig. 3.25
Discontinuous p63 expression in basal cells of atypical adenomatous hyperplasia (adenosis) of the prostate
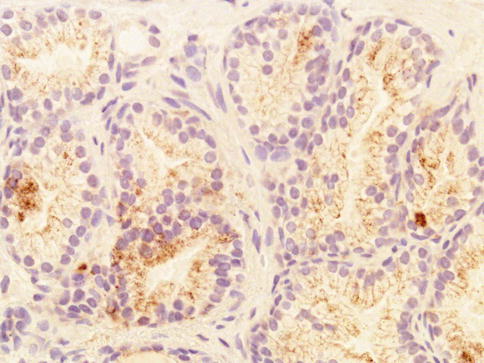
Fig. 3.26
Weak racemase expression in secretory cells of atypical adenomatous hyperplasia (adenosis) of the prostate
3.2.1.4 Post-inflammatory Atrophy (PIA)
Recent reports suggest that post-inflammatory atrophy, may be causally linked to PIN and therefore to prostate adenocarcinoma. This hypothesis is based upon recognition of increased proliferative activity, albeit low, in the secretory cells that persist within atrophic acini.
3.2.1.5 Atypical Small Acinar Proliferation Suspicious for But Not Diagnostic of Malignancy
Atypical focus suspicious but not diagnostic of malignancy (ASAP), also referred to as atypical small acinar proliferation suspicious for but not diagnostic of malignancy or just atypical prostate glands (ATYP), is not a preneoplastic lesion. It is descriptive diagnostic terminology used in the pathology report of a needle biopsy containing a small group of glands suspicious for adenocarcinoma, however with insufficient cytological and/or architectural atypia to establish a definitive diagnosis. In some cases (16–31 % ) the lesion is associated with PIN (PIN ASAP or PIN ATYP).
It is a broad diagnostic category that encompasses benign lesions mimicking malignant glandular proliferations and under sampled, small foci of carcinoma that harbour some but not all of the features needed for a definitive diagnosis of malignancy. It is not a diagnostic entity and is not synonymous with high-grade prostatic intraepithelial neoplasia (HGPIN).
The widespread use of basal cell immunohistochemistry using antibodies against p63 or 34βE12 or the use of racemase (P504S) turned the use of the diagnostic term ASAP uncommon nowadays (Figs. 3.27 and 3.28; Table 3.2).
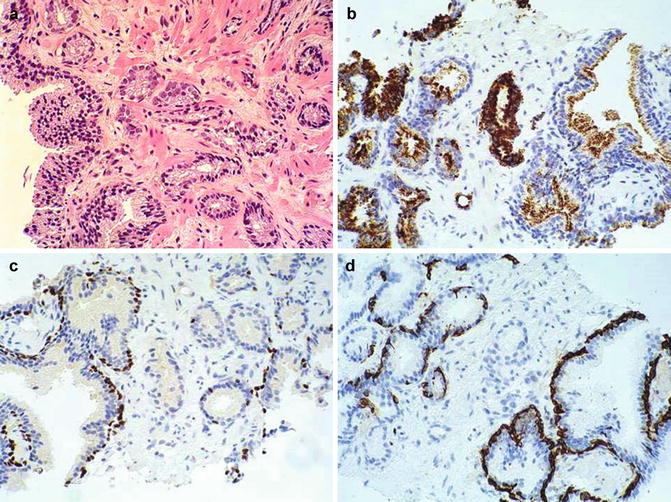
Fig. 3.27
Histologic features of atypical small acinar proliferation. (a) H&E; (b) Racemase positive; (c) p63 positive in a few basal cells; (d) Negative basal cell cytokeratin. In this scenario, the presence of p63 positive basal cells suggest that the lesion might be related to a PIN with invasion more than a carcinoma
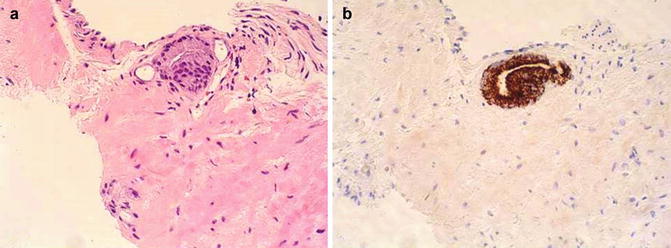
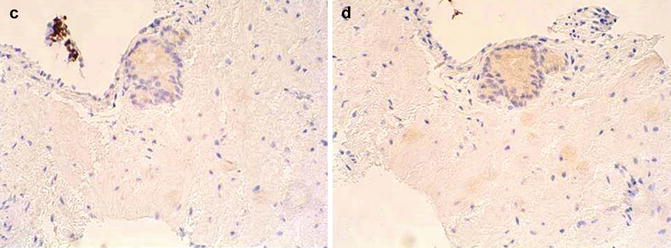
Fig. 3.28
Histologic features of atypical small acinar proliferation. (a) H&E; (b) Racemase positive; (c) Negative basal cell cytokeratin; (d) Negative p63. In this scenario, the absence of basal cells and the strong positive for racemase support malignancy even in a limited specimen
Table 3.2
Factors resulting in the diagnosis of atypical small acinar proliferations suspicious for malignancy
Small size of focus
Small number of acini in the focus of concern (invariably fewer than two dozen acini)
Small focus size, average 0.4 mm in diameter
Focus at core tip or biopsy edge, indicating that the focus is incompletely sampled
Loss of focus of concern in deeper levels
Conflicting morphologic findings
Distortion of acini raising concern for atrophy
Lack of convincing features of cancer (insufficient nucleomegaly or nucleolomegaly)
Clustered growth pattern mimicking a benign process such as atypical adenomatous hyperplasia
Foamy cytoplasm raising concern for foamy gland carcinoma
Conflicting immunohistochemical findings
Focally positive high molecular weight cytokeratin
Focally positive p63 staining
Negative racemase immunostain
Confounding findings
Histologic artifacts such as thick sections or overstained nuclei
Tangential cutting of adjacent high-grade PIN
Architectural or cytologic changes (nuclomegaly and nucleolomegaly) owing to inflammation or other lesions
3.3 Diagnostic Criteria for Prostate Adenocarcinoma
Most clinically palpable prostate cancers diagnosed on needle biopsy are predominantly located posterolateral. Large transition zone tumours may extend into the peripheral zone and become palpable.
Cancers detected on TURP are predominantly within the transition zone. Non-palpable cancers detected on needle biopsy are predominantly located peripherally, although 20 % have tumour predominantly within the transition zone. Large tumours may extend into the central zone, yet cancers uncommonly arise in this zone. Multifocal adenocarcinoma of the prostate is seen in about 85 % of prostates.
Grossly evident cancers are firm, solid, and range in colour from white-grey to yellow-orange, the latter having increased cytoplasmic lipid; the tumours contrast with the adjacent benign parenchyma, which is typically tan and spongy. Tumours usually extend microscopically beyond their macroscopic border.
Gross haemorrhage and necrosis are rare. Subtle tumours may be grossly recognized by structural asymmetry; for example, peripheral zone tumours may deform the periurethral fibromuscular concentric band demarcating the periurethral and peripheral prostate centrally, and peripherally may expand or obscure the out of boundaries of the prostate. Anterior and apical tumours are difficult to grossly identify because of admixed stromal and nodular hyperplasia.
Grossly recognizable tumours tend to be larger, of higher grade and stage, and are frequently palpable, compared with grossly unapparent tumours (usually <5 mm) which are often non-palpable, small, low grade and low stage. Some large tumours are diffusely infiltrative, and may not be evident grossly.
In countries with widespread PSA testing, grossly evident prostate cancer has become relatively uncommon (Table 3.3).
Table 3.3
Diagnostic criteria for prostatic carcinoma
Architectural features
Infiltrative pattern of malignant acini, e.g.:
Arrangement: irregular and haphazard
Spacing between acini varies widely
Variation in size
Irregular contour
Basal cell layer
Absent
Cytological features (secretory cells in single layer)
Nuclear hyperchromasia
Nuclear enlargement
Nucleolar enlargement or prominence
Mitotic figures
Amphophilic cytoplasm
Luminal findings
Crystalloids
Intraluminal blue mucin and/or pink amorphous secretions
Stromal findings
Collagenous micronodules
Immunohistochemical findings
Prostate-specific antigen
Prostatic acid phosphatase
34βE12
p63
Racemase
Additional feature
Adjacent prostatic intraepithelial neoplasia
3.3.1 Histologic Features of Prostate Cancer
The diagnosis of carcinoma relies on a combination of architectural and cytological findings. Greater than 95 % of all carcinomas of the prostate seen in needle biopsy specimens are referred to as acinar or conventional type.
3.3.1.1 Architectural Features
Architectural features are usually assessed at low to medium power magnification, with emphasis on spacing, size, and shape of acini. The arrangement of the acini is diagnostically useful and is the basis of the Gleason grade.
Malignant acini usually have an irregular, haphazard arrangement, randomly scattered in the stroma in clusters or singly. The spacing between malignant acini often varies widely. Variation in acinar size is a useful criterion for cancer, particularly when small, irregular, abortive acini with primitive lumens are seen at the periphery of a focus of well-differentiated carcinoma (Fig. 3.29)
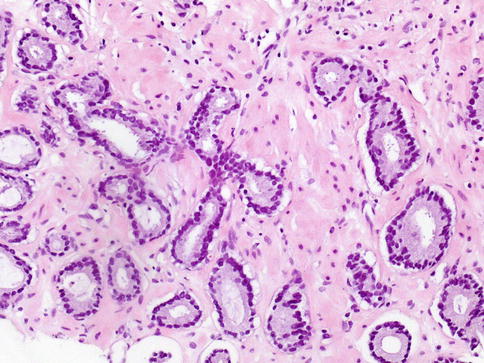
Fig. 3.29
Microscopic appearance of acinar (conventional) prostate cancer showing infiltrating acini, amphophilic cytoplasm, retraction artefact of the glands, and minimal nuclear atypia
The acini in suspicious foci are usually small or medium sized, with irregular contours that contrast with the typical smooth, round to elongated contours of benign and hyperplastic acini. Comparison with the adjacent benign prostatic acini is always of value in the diagnosis of cancer (Fig. 3.30)
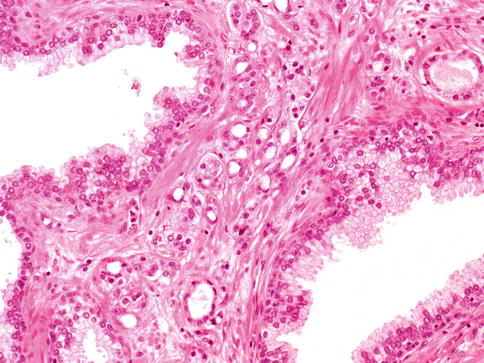
Fig. 3.30
Infiltrating acini between non-malignant ducts. Comparison with the adjacent benign prostatic acini is of value in the diagnosis of cancer
Well-differentiated carcinoma and the large acinar variant of Gleason 3 carcinoma are particularly difficult to separate from benign acini in needle biopsies because of the uniform size and spacing of acini; in such cases, greater emphasis is placed on cytologic features, immunohistochemical findings, and the presence of smaller diagnostic acini at the edge of the focus (Figs. 3.31, 3.32, 3.33 and 3.34).
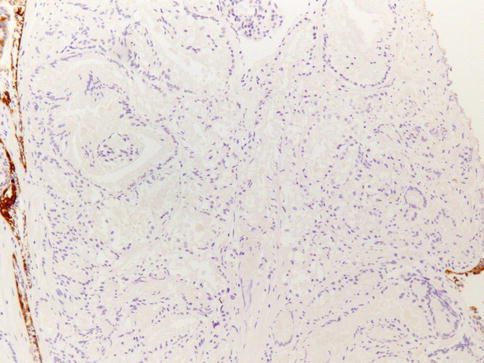
Fig. 3.31
Basal cells are typically lost in prostate carcinoma. Anti-34BE12 immunohistochemistry
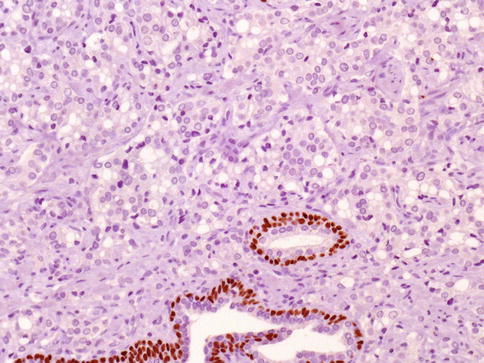
Fig. 3.32
Basal cells are typically lost in prostate carcinoma. Anti-p63 immunohistochemistry
Fig. 3.33
Rare cases of prostate carcinoma may be positive with current anti-p63 antobodies, a challenging diagnostic factor
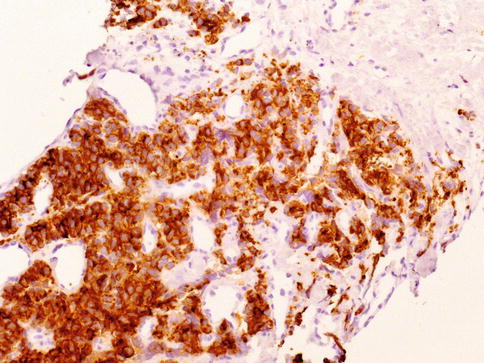
Fig. 3.34
Strong cytoplasmic expression of racemase is a characteristic of acinar prostate carcinoma
The basal cell layer is absent in prostate cancer, whereas an intact basal cell layer is present with benign acini. This is an important diagnostic feature that is not always easy to evaluate in routine tissue sections owing to false negative findings with atrophy and other mimics of cancer. Compressed stromal fibroblasts may mimic basal cells but are usually seen only focally at the periphery of acini.
3.3.1.2 Cytological Features
The cytological features of adenocarcinoma include nuclear and nucleolar enlargement, which occurs in most malignant cells. Every cell has a nucleolus at least 1.50 μm in diameter or larger. Artifacts such overstaining and differences in fixation often obscure the nuclei and nucleoli, so comparison with non-neoplastic cells (internal control) from the same specimen is a useful diagnostic clue (Figs. 3.35 and 3.36).
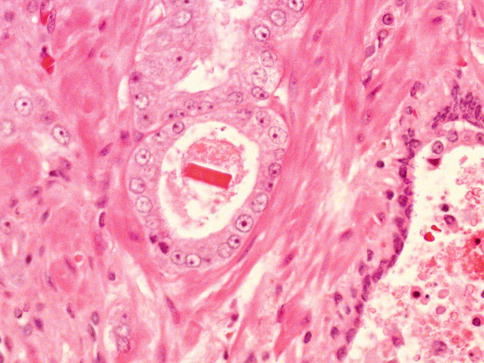
Fig. 3.35
Prominent nucleolus is characteristic of many prostate carcinomas. In this case, luminal crystals are also present
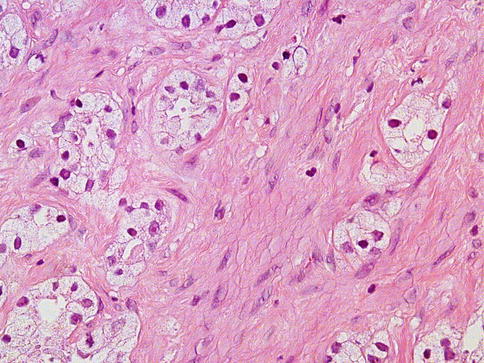
Fig. 3.36
Prominent nucleolus may not be present in some cases. This case shows clear-type cytoplasm and nucleolus-poor features
3.3.1.3 Luminal Findings
Crystalloids are sharp, needle-like eosinophilic structures that are often present in the lumens of well-differentiated and moderately differentiated carcinoma. They are not specific for carcinoma and can be found in other conditions. The presence of crystalloids in metastatic adenocarcinoma of unknown site of origin is strong presumptive evidence of prostatic origin, although it is an uncommon finding (Fig. 3.37).
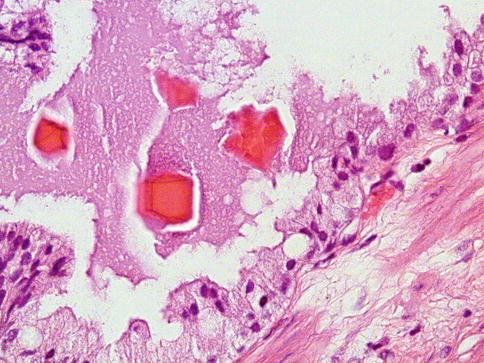
Fig. 3.37
Some prostate carcinoma may present luminal basophilic material and crystals
Luminal acidic sulfated and nonsulfated mucin is often seen in acini of adenocarcinoma, appearing as amorphous, faintly basophilic secretions in routine sections. This mucin stains with Alcian blue at pH2.5, whereas normal prostatic epithelium contains periodic acid Schiff-reactive mucin that is neutral (Fig. 3.38).
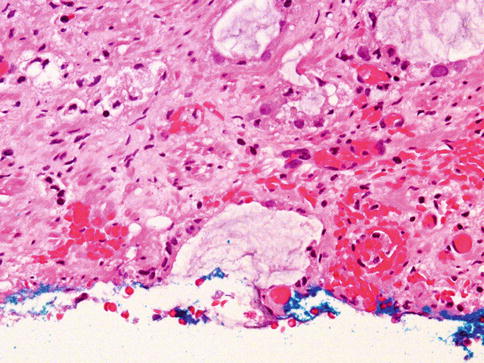
Fig. 3.38
Mucin is also present in some prostate carcinoma glands
Acidic mucin is not specific for carcinoma. It may be found in prostatic intraepithelial neoplasia (PIN), atypical adenomatous hyperplasia, sclerosing adenosis, and, rarely, nodular hyperplasia.
Intraluminal corpora amylacea may be seen occasionally in prostate cancer.
3.3.1.4 Stromal Findings
The stroma in cancer frequently contains young collagen, which appears lightly eosinophilic, although desmoplasia may be prominent. Muscle fibers in the stroma are sometimes split or distorted. But most frequently resembles the stroma associated with benign prostate (Fig. 3.39).
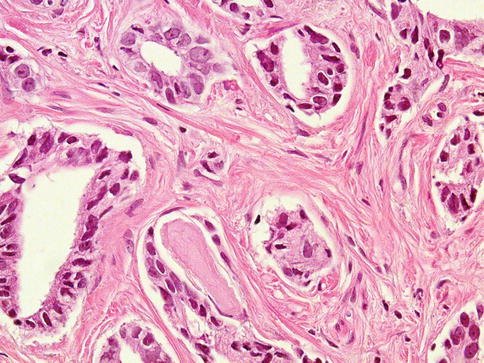
Fig. 3.39
Prostate carcinoma with prominent fibroblastic stroma
Collagenous micronodules (mucinous fibroplasia) are a specific but infrequent and incidental finding in prostatic adenocarcinoma. They consist of microscopic nodular masses of paucicellular eosinophilic fibrilar stroma that impinge on acinar lumens. They are usually present in mucin-producing adenocarcinoma and result from extravasation of acidic mucin into the stroma (Fig. 3.40).
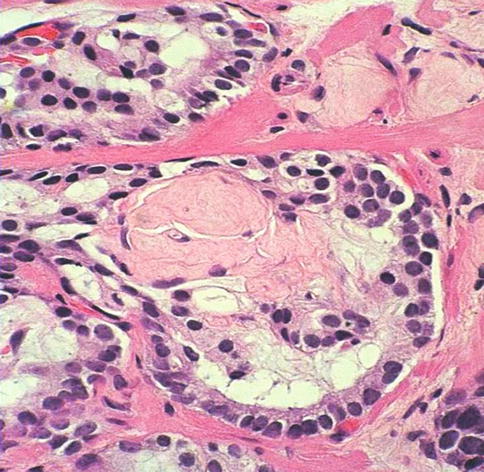
Fig. 3.40
Collagenous micronodules are a an uncommon feature of prostate carcinoma
Collagenous micronodules are present in about 14 % of cases of adenocarcinoma. Collagenous micronodules may be particularly valuable in challenging needle biopsy specimens. They are not observed in PIN, nodular hyperplasia, or benign epithelium.
3.3.1.5 Immunohistochemical Findings
Important immunohistochemical markers in prostate pathology are PSA, PAP, PSMA, high-molecular-weight keratin clone 34βE12, CK 5/6, p63 and AMACR.
Immunohistochemical expression of Prostate-Specific Antigen (PSA) is useful for distinguishing high-grade prostate cancer from urothelial carcinoma, colonic carcinoma, granulomatous prostatitis, and lymphoma. PSA also facilitates identification of the site of tumour origin in metastatic adenocarcinoma. PSA can be detected in frozen and paraffin-embedded sections and is preserved in decalcified specimens. Staining is invariably heterogeneous.
The use of PSMA adds specificity over PSA according to some authors. Prostatic Acid Phosphatase (PAP) is a valuable immunohistochemical marker for identifying prostate cancer when used in combination with PSA.
High-molecular-weight cytokeratin (clone 34βE12) and CK5/6
May be useful for evaluation of the basal cell layer. It is most useful in confirming the benignancy of a suggestive focus by showing an immunoreactive basal cell layer. Anti-keratin 34βE12 stains nearly all of the normal basal cells of the prostate; no staining occurs in the secretory and stromal cells.
Uniform absence of a basal cell layer is one important diagnostic feature of invasive carcinoma and basal cells may be inapparent by Hematoxilin-Eosin (H&E) stain, basal cell specific immunostains may help to distinguish invasive prostatic adenocarcinoma from benign small acinar cancer mimics which retain their basal cell layer, e.g. glandular atrophy, post-atrophic hyperplasia, atypical adenomatous hyperplasia, sclerosing adenosis and radiation induced atypia.
Because the basal cell layer may be interrupted or not demonstrable in small numbers of benign glands, the complete absence of a basal cell layer in a small focus of acini cannot be used alone as a definitive criterion for malignancy; rather, absence of a basal cell layer is supportive of invasive carcinoma only in acinar proliferations which exhibit suspicious cytological and/or architectural features on H&E stain.
Conversely, some early invasive prostatic carcinomas, e.g. microinvasive carcinomas arising in association with or independent of high grade prostatic intraepithelial neoplasia, may have residual basal cells (transitive glands). Intraductal spread of invasive carcinoma and entrapped benign glands are other proposed explanations for residual basal cells. Rare prostatic adenocarcinomas contain sparse neoplastic glandular cells which are immunoreactive for 34βE12, yet these are not in a basal cell distribution. The use of antibodies for 34βE12 is especially helpful for the diagnosis for of deceptively being appearing variants of prostate cancer.
Immunohistochemistry for cytokeratins 7 and 20 have a limited diagnostic use in prostate pathology with the exception that negative staining for both markers, which can occur in prostate adenocarcinoma, would be unusual for transitional cell carcinoma.
p63
A nuclear protein encoded by a gene on chromosome 3q27-29 with homology to p53 (a tumour suppressor gene), has been shown to regulate growth and development in epithelium of the skin, cervix, breast and urogenital tract.
Specific isotypes are expressed in basal cells of stratified and pseudostratified epithelia (prostate, bronchial), reserve cells of simple columnar epithelia (endocervical, pancreatic ductal), myoepithelial cells (breast, salivary glands, cutaneous apocrine/eccrine glands), urothelium and squamous epithelium. p63 has similar applications to those of high molecular weight cytokeratins in the diagnosis of prostatic adenocarcinoma, but with the advantages that p63 is less susceptible to the staining variability of 34βE12 (particularly in TURP specimens with cautery artefact), stains a subset of 34βE12 negative basal cells, and is easier to interpret because of its strong nuclear staining intensity.
Interpretative limitations related to presence or absence of basal cells in small numbers of glands for 34βE12 apply to p63, requiring correlation with morphology. Prostatic adenocarcinomas have occasional p63 immunoreactive cells, most representing entrapped benign glands or intraductal spread of carcinoma with residual basal cells, but some true p63 positive carcinomas have been reported.
An immunohistochemical cocktail containing monoclonal antibodies to cytokeratin 34betaE12 and p63 is in use by some authorities.
AMACR (α-Methyl-CoA Racemase, P504S)
This mRNA was found to encode a racemase protein, for which polyclonal and monoclonal antibodies have been produced which are active in formalin-fixed, paraffin- embedded tissue. Over 80 % of prostatic adenocarcinomas are labelled with racemase.
Certain subtypes of prostate cancer, such as foamy gland carcinoma, atrophic carcinoma, pseudohyperplastic, and treated carcinoma show lower AMACR expression. However, AMACR is not specific for prostate cancer and is present in nodular hyperplasia (12 %), atrophic glands (35 %), HGPIN (90 %) and atypical adenomatous hyperplasia (18 %).
AMACR may be used as a confirmatory stain for prostatic adenocarcinoma, in conjunction with histology and basal cell specific markers. AMACR is expressed in other non-prostatic neoplasms including urothelial, renal and colon cancer.
A cocktail containing 34betaE12, p63 and racemase is used by some authors.
3.3.1.6 Focal Neuroendocrine Differentiation
About 10 % of prostatic carcinomas have zones with a large number of single or clustered neuroendocrine cells detected by chromogranin A immunohistochemistry, some cells may also be serotonin positive. Immunostaining for neuron-specific enolase, synaptophysin, bombesin/gastrin-releasing peptide and a variety of other neuroendocrine peptides may also occur in individual neoplastic neuroendocrine cells, or in a more diffuse pattern and receptors for serotonin may also be present (Fig. 3.41).
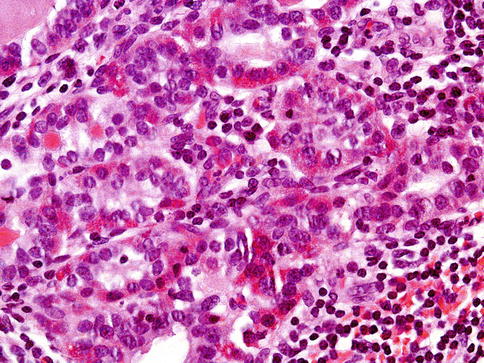
Fig. 3.41
Focally prominent of neuroendocrine cells (Paneth cell-like change) is an uncommon finding in prostate cancer
There are conflicting studies as to whether advanced androgen independent and androgen deprived carcinomas show increased neuroendocrine differentiation. The prognostic significance of focal neuroendocrine differentiation in primary untreated prostatic carcinoma is controversial.
In advanced prostate cancer, especially androgen independent cancer, focal neuroendocrine differentiation portends a poor prognosis.
3.4 Rare Histologic Subtypes of Prostatic Carcinoma
A wide variety of histologic subtypes and variants of adenocarcinoma may be seen in the prostate (Table 3.4).
Table 3.4
Histological classification of carcinoma of the prostate
1. Adenocarcinoma (acinar, conventional, usual type)
2. Variants of adenocarcinoma and other carcinomas
Pseudohyperplastic adenocarcinoma
Foamy gland adenocarcinoma
Atrophic adenocarcinoma
Adenocarcinoma with glomeruloid features
Mucinous (colloid) adenocarcinoma
Signet ring cell carcinoma
Oncocytic adenocarcinoma
Lymphoepithelioma-like carcinoma
PIN-like
Pleomorphic giant cell carcinoma
Prostatic duct adenocarcinoma
Small cell carcinoma and other neuroendocrine tumours
Sarcomatoid carcinoma
Basal cell carcinoma
Urothelial carcinoma
Adenosquamous carcinoma
Squamous cell carcinoma
3.4.1 Pseudohyperplastic Adenocarcinoma
Pseudohyperplastic prostate cancer resembles benign prostate glands in that the neoplastic glands are large with branching and papillary infolding. Most occur in the transition zone which is the usual site for benign prostatic hyperplasia (Fig. 3.42).
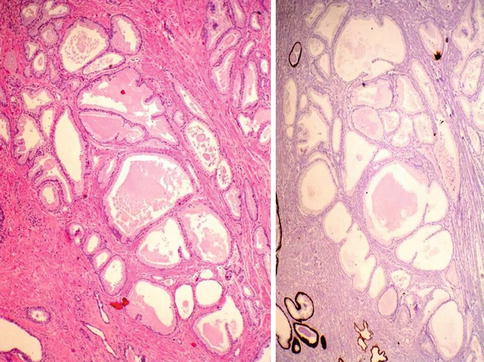
Fig. 3.42
Pseudohyperplastic carcinoma may simulate benign hyperplasia of the prostate (left) but lack basal cell layer (right)
The recognition of cancer with this pattern is based on the architectural pattern of numerous closely packed glands as well as nuclear features more typical of carcinoma. Pseudohyperplastic cancer, it is considered Gleason score 3 + 3 = 6.
One pattern of pseudohyperplastic adenocarcinoma consists of numerous large glands that are almost back-to-back with straight even luminal borders, and abundant cytoplasm. Comparably sized benign glands either have papillary infolding or are atrophic.
The presence of cytological atypia in some of these glands further distinguishes them from benign glands. It is helpful to verify the absence of basal cells by immunohistochemistry.
HOXB13 G84E variant-related prostate cancers show frequent pseudohyperplastic-type features and markedly low prevalence of ERG.
A cystic variant of pseudohyperplastic carcinoma (microcystic carcinoma) has been recognized.
3.4.2 Foamy Gland Adenocarcinoma
Foamy gland (also known as xanthomatous) cancer is a variant of acinar adenocarcinoma of the prostate that is characterized by having abundant foamy appearing cytoplasm. Although the cytoplasm has a xanthomatous appearance, it does not contain lipid, but rather empty microvacuoles (Figs. 3.43 and 3.44).
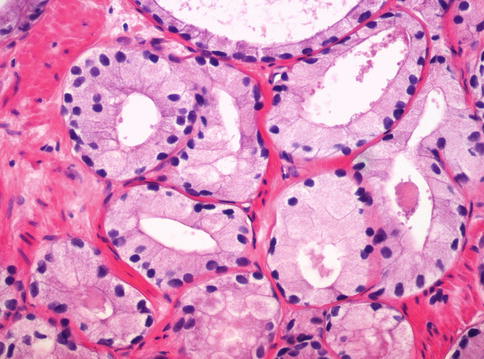
Fig. 3.43
Foamy gland adenocarcinoma showing xanthomatous cytoplasm of the cells
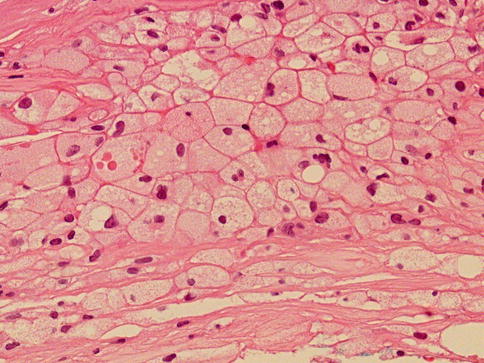
Fig. 3.44
Prostate xanthoma (this case) to be compared with foamy gland adenocarcinoma
Typical features of adenocarcinoma such as nuclear enlargement and prominent nucleoli are frequently rare to absent, which makes this lesion difficult to recognize as carcinoma especially on biopsy material.
Whereas most cases of foamy gland carcinoma would be grade as Gleason 3 + 3, higher grade foamy gland carcinoma exists and should be graded accordingly based on the pattern; most frequently Gleason pattern 4.
Characteristically, the nuclei in foamy gland carcinoma are small and densely hyperchromatic. Nuclei in foamy gland cancer are round, more so than those of benign prostatic secretory cells. It is recognized as carcinoma by its architectural pattern of crowded and/or infiltrative glands, and frequently present dense pink acellular secretions.
In most cases, foamy gland cancer is seen in association with ordinary adenocarcinoma of the prostate.
3.4.3 Atrophic Adenocarcinoma
An unusual variant of prostate cancer resembles benign atrophy owing to its scant cytoplasm. Atrophic prostate cancers are unrelated with a prior history of treatment (Fig. 3.45).
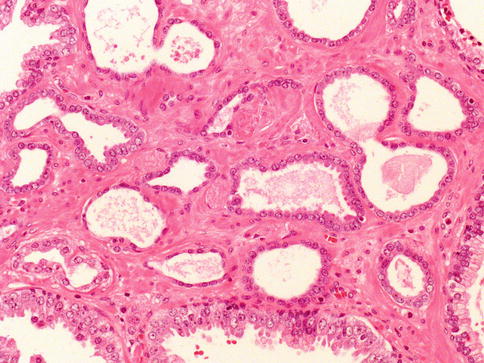
Fig. 3.45
Microscopic appearance of atrophic prostate carcinoma
The diagnosis is based on several features. First, atrophic prostate cancer may demonstrate a truly infiltrative process with individual small atrophic glands situated between larger benign glands. In contrast, benign atrophy has a lobular configuration with a centrally dilated atrophic gland surrounding by clustered smaller glands that are not truly infiltrative (Fig. 3.46).
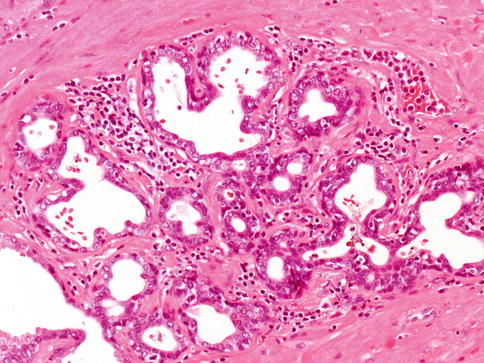
Fig. 3.46
Atrophic cancer needs to be differentiated from non-neoplastic atrophic glands. In this case lobular atrophy
Whereas some forms of atrophy, are associated with fibrosis, atrophic prostate cancer lack fibrotic stroma. Atrophic prostate cancer may also be differentiated from benign atrophy by the presence of marked cytological atypia. Atrophy may show enlarged nuclei and prominent nucleoli, although not the huge eosinophilic nucleoli seen in some atrophic prostate cancers.
Ordinary type carcinoma is usually present. The absence of basal cell layer at immunohistochemistry is of help. AMACR is expressed in 70 % of atrophic carcinomas, which is lower compared to acinar adenocarcinoma.
Most glands are well formed with lumina (Gleason 3), and less often exhibit fusion (Gleason 4).
Main differential diagnosis is simple atrophy which shows a lobular growth and does not exhibit gland infiltration. Benign atrophic glands retain basal cell layer that may be discontinuous and atrophic cancer has a complete loss of basal cell layer.
3.4.4 Adenocarcinoma with Glomeruloid Features
Prostatic adenocarcinoma with glomeruloid features is characterized by intraluminal ball-like clusters of cancer cells reminiscent of renal glomeruli. Glomeruloid structures in the prostate represent an uncommon but distinctive pattern of growth that is specific for malignancy (glomeruloid-like structures in non-malignant glands are extremely rare) (Fig. 3.47).
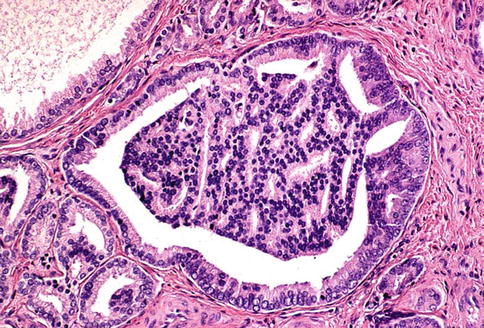
Fig. 3.47
Microscopic appearance of glomeruloid prostate cancer
Glomeruloid features may be a useful diagnostic clue for malignancy, particularly in some challenging needle biopsy specimens. This pattern of growth is usually seen in high-grade adenocarcinoma, often with extraprostatic extension. A Gleason score of 4 has been suggested for this variant of prostate cancer.
3.4.5 Mucinous and Signet Ring Cell Adenocarcinoma
Mucinous adenocarcinoma is one of the least common morphologic variants of prostatic carcinoma. The diagnosis of mucinous (colloid) adenocarcinoma of the prostate gland should be made when at least 25 % of the tumour resected contains lakes of extracellular mucin.
Mucinous prostate adenocarcinomas behave aggressively with most patients died or were alive with disease 5 years after therapy. Mucinous prostate adenocarcinomas have a propensity to develop bone metastases. These cases should be graded as Gleason score 4 + 4 (Fig. 3.48).
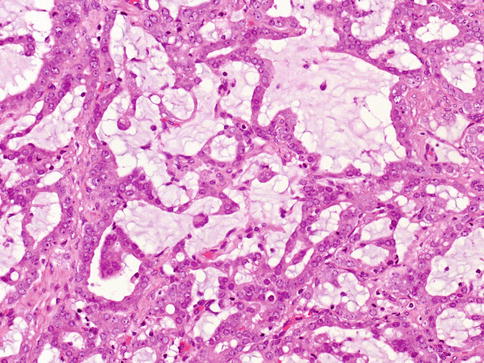
Fig. 3.48
Microscopic appearance of mucinous (colloid) adenocarcinoma
Mucinous adenocarcinoma shows higher (83 %) incidence of TMPRSS2-ERG fusion that usual acinar carcinoma (about 50 %).
Some carcinomas of the prostate may have a signet-ring-cell appearance, yet the vacuoles do not contain mucin (prostatic carcinoma with cytoplasmic vacuoles) (Fig. 3.49). These vacuolated cells may be present as singly invasive cells, in single glands, and in sheets of cells. These tumors should be graded as if the vacuoles were not present.
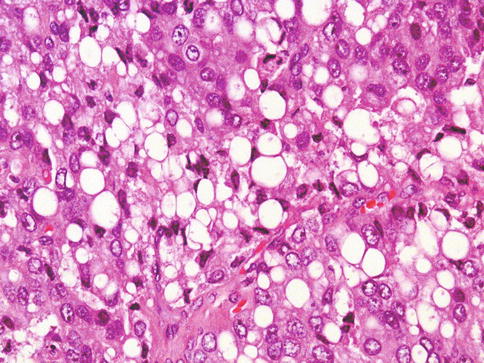
Fig. 3.49
Microscopic appearance of prostate carcinoma with cytoplasmic vacuoles (signet ring-like). This need to be separated from mucinous signet-ring carcinoma
Only a few cases of prostate cancer have been reported with mucin positive signet cells (signet ring mucinous adenocarcinoma). These tumors seem to be very aggressive., and should be distinguished from adenocarcinoma with cytoplasmic vacuoles (see above).
3.4.6 Oncocytic Adenocarcinoma
This is an exceptionally rare variant of PCa with unclear clinical significance (Fig. 3.50).
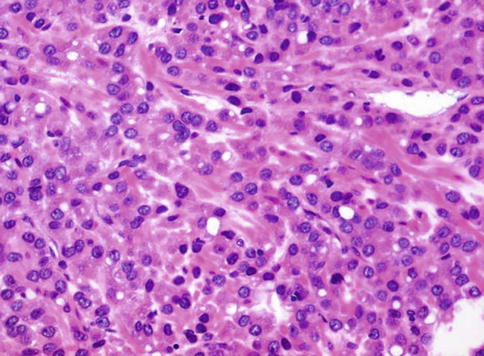
Fig. 3.50
Microscopic appearance of oncocytic prostate carcinoma
Tumour cells have round to ovoid hyperchromatic nuclei, and have granular cytoplasm with numerous mitochondria on ultrastructural or immunohistochemical examination and are strongly positive for PSA. A high Gleason grade, elevated serum PSA and metastasis of similar morphology have been documented.
3.4.7 Lymphoepithelioma-Like Carcinoma of the Prostate
This undifferentiated carcinoma is characterised by indistinct cytoplasmic borders with syncytial arrangement of malignant cells associated with a heavy lymphocytic infiltrate including some plasma cells and neutrophils; one case had a prominent infiltration of eosinophils. Associated ordinary adenocarcinoma is always present and may be of acinar, adenosquamous or ductal type (Figs. 3.51, 3.52 and 3.53).
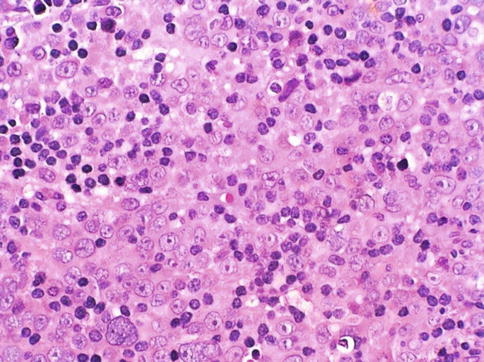
Fig. 3.51
Microscopic appearance of lymphoepithelioma-like carcinoma of the prostate
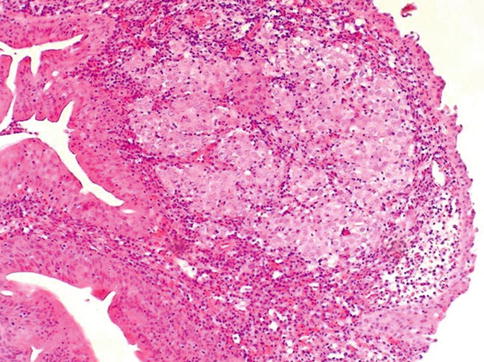
Fig. 3.52
Lymphoepithelioma-like carcinoma may also occur in the prostatic urethra
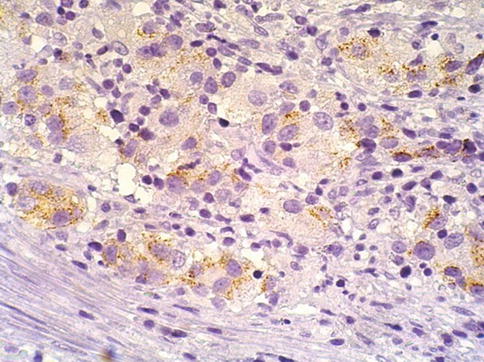
Fig. 3.53
Weak racemase positive expression in lymphoepithelioma-like carcinoma of the prostate
Immunohistochemical staining demonstrated that lymphoepithelioma-like carcinoma was positive for prostate-specific antigen, prostate acid phosphatase, alpha-methylacyl coenzyme A racemase, and epithelial membrane antigen; several cytokeratins (AE1/AE3, 7, 8, and 20 [rare cells]) were also immunoreactive. The mean Ki-67 labeling index was 53 % (range, 40–0 %), and the p53 expression in all cases was low (10–20 %).
The lymphoid component was mainly composed of T with a minor subset of B cells, admixed with some dendritic cells and histiocytes as seen by S100 and CD68 immunoreactivity. Latent membrane protein 1 immunostaining and in situ hybridization for Epstein-Barr virus were negative in all 5 lymphoepithelioma-like carcinoma cases.
DNA ploidy of lymphoepithelioma-like carcinoma tumors gave DNA histograms with aneuploid peaks. DNA ploidy of the concurrent adenocarcinoma gave DNA aneuploid peaks except in one DNA diploid case.
The largest series included 5 patients at a mean age of 76 years with obstructive symptoms and high PSA. Most presented at advanced clinical stage. Four patients died of disease from 8 to 26 months.
3.4.8 Prostatic Ductal Adenocarcinoma
Subtype of adenocarcinoma (also known as endometrioid carcinoma) composed of larger glands lined by tall pseudostratified columnar cells. It was originally thought to originate from prostate utricle. In pure form, ductal adenocarcinoma accounts for 0.2–0.8 % of prostate cancers. It is assumed that almost all cases have an acinar component (Figs. 3.54, 3.55, 3.56, 3.57, 3.58, 3.59 and 3.60).
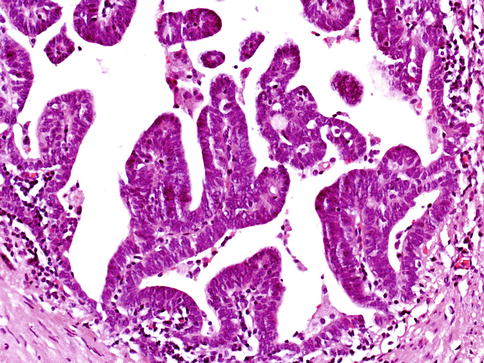
Fig. 3.54
Microscopic appearance of ductal carcinoma of the prostate with endometrioid features
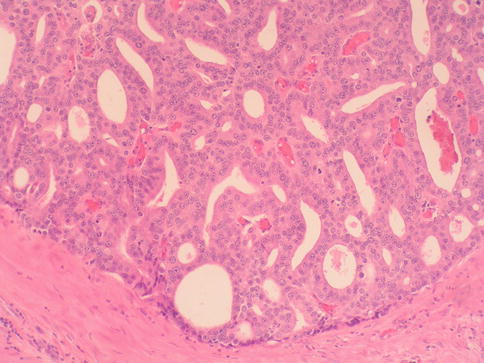
Fig. 3.55
Microscopic appearance of ductal carcinoma of the prostate with cribriform features
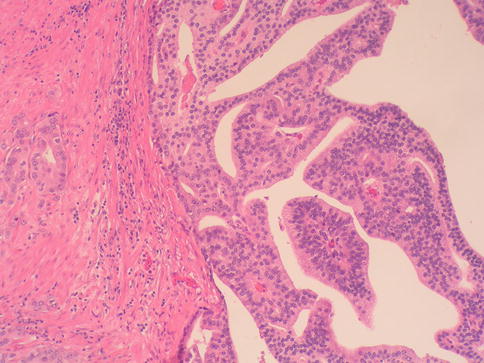
Fig. 3.56
Microscopic appearance of ductal carcinoma of the prostate with papillary features
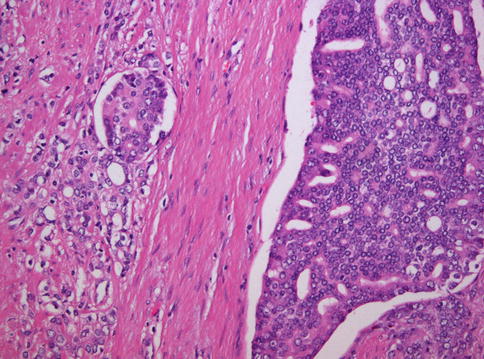
Fig. 3.57
Microscopic appearance of ductal carcinoma of the prostate with cribriform features (right) and associated acinar carcinoma (left)
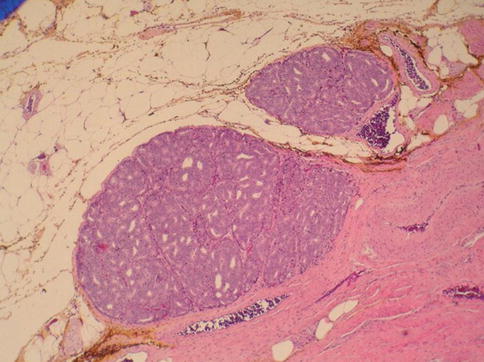
Fig. 3.58
Ductal carcinoma with extraprostatic extension
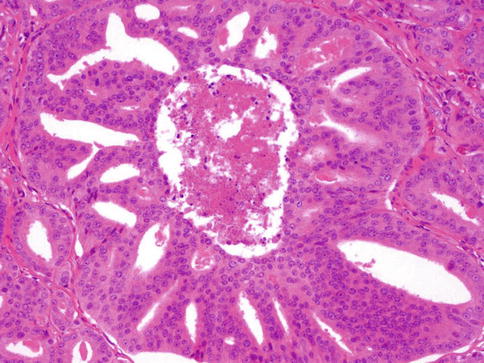
Fig. 3.59
Ductal carcinoma with comedo-type necrosis
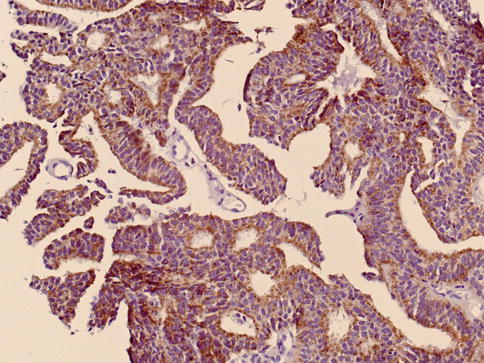
Fig. 3.60
Racemase expression in ductal carcinoma of the prostate
Ductal adenocarcinoma may be located centrally around the prostatic urethra or more frequently located peripherally admixed with typical acinar adenocarcinoma. A centrally located adenocarcinoma may also be associated with a peripherally located acinar adenocarcinoma. Peripherally occurring ductal carcinomas typically show a white-gray firm appearance similar to acinar adenocarcinoma. These tumors may lead to enlargement or induration of the prostate.
Centrally occurring tumours appear as exophytic polypoid or papillary masses protruding into the urethra around the verumontanum and may cause hematuria, urinary urgency and eventually urinary retention. In these cases, there may be no abnormalities on rectal examination. Because of the papillary growth, some of these cases may be misdiagnosed as papillary urothelial carcinoma of high grade arising in the urethra.
Of importance is that serum PSA levels may be normal particularly in patients with only centrally located tumors. In most cases, transurethral resections performed for diagnosis or relief of the urinary obstruction will provide an accurate diagnostic. Transrectal needle core biopsies may also obtain diagnostic tissue when the tumour is more peripherally located. In addition, areas of ductal adenocarcinoma may be incidentally identified in prostatectomy specimens. And in those cases there is a tendency to find more advanced disease.
A Gleason score of 4 + 4 (or 9 if comedonecrosis is present) applies to ductal prostatic adenocarcinoma. The recent described PIN-like ductal carcinoma should be graded as Gleason pattern 3 (Fig. 3.61).
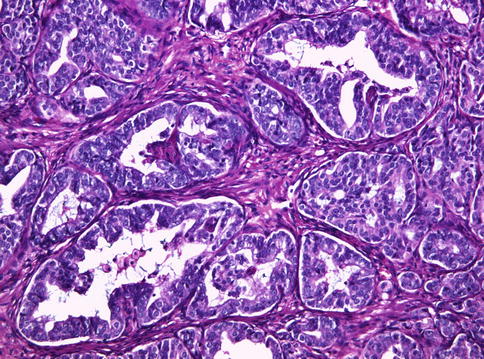
Fig. 3.61
Histologic features of PIN-like ductal carcinoma of the prostate
Histology and Immunohistochemistry
Ductal adenocarcinoma is characterized by tall columnar cells with abundant usually amphophilic cytoplasm, which form a single or pseudostratified layer. The cytoplasm of is often amphophilic and may occasionally appear clear. In some cases, there are numerous mitoses and marked cytological atypia. In other cases, the cytological atypia is minimal, which makes a diagnosis difficult particularly on needle biopsy. Peripherally located tumours are often admixed with cribriform, glandular or solid patterns as seen in acinar adenocarcinoma.
Ductal adenocarcinomas are mostly equivalent to Gleason patterns 4. In some cases comedo-type of necrosis is present and therefore considered equivalent to Gleason pattern 5.
Ductal adenocarcinoma displays a variety of architectural patterns which are often intermingled: cribriform, papillary, individual gland or solid pattern. Immunohistochemically ductal adenocarcinoma is strongly positive for PSA and PAP and racemase (Fig. 3.60). Tumour cells are typically negative for basal cell specific markers; however, a frequently incomplete basal cell layer may be present in pre-existing ducts with cancer.
Ductal adenocarcinoma usually spread along the urethra or into the prostatic ducts with or without stromal invasion, or may spread similar to that of acinar carcinoma with invasion to extraprostatic tissues and metastasis to pelvic lymph nodes or distal organs. Ductal adenocarcinomas appear to have a tendency to metastasize to lung and penis, but also may metastasize to bones.
Differential diagnosis and prognosis
Ductal adenocarcinoma must be distinguished from urothelial carcinoma, ectopic prostatic tissue, benign prostatic polyps, and papillary-polypoid urethritis.
Ductal adenocarcinoma is aggressive with 27–39 % of cases showing metastases at the time of diagnosis. Five-year survival rate is poor ranging 14–40 % of patients.
3.4.9 Intraductal Carcinoma
Intraepithelial spread of prostatic carcinoma within pre-existing non-neoplastic ducts or acini is known as intraductal carcinoma (IDC). It is characterized by the preservation of basal cells and is now regarded as tumor progression prior to cancer invasion. Other suggests that is a carcinomatous evolution of high grade PIN preceding invasion (Figs. 3.62, 3.63 and 3.64).
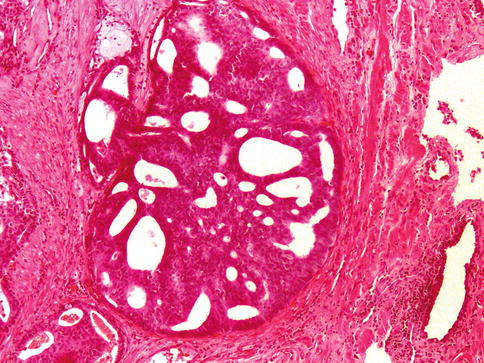
Fig. 3.62
Intraductal carcinoma of the prostate. Microscopic features
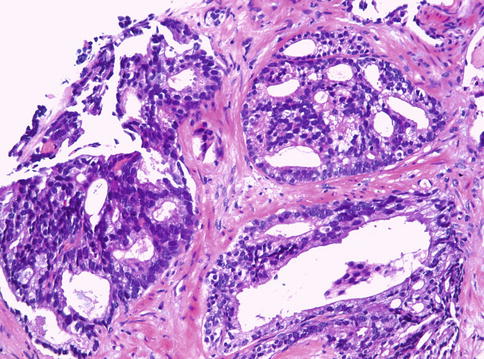
Fig. 3.63
Intraductal carcinoma of the prostate as seen in needle biopsy
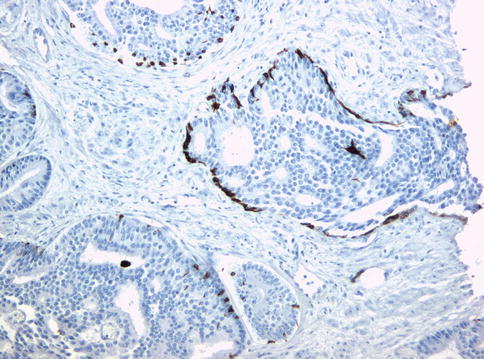
Fig. 3.64
Basal cells are still present in intraductal carcinoma as seen with antibodies against basal cell cytokeratins
Diagnosis of IDC in practice is limited by its problematic distinction from PIN; both lesions retain basal cell as seen by immunohistochemistry. Most cases are seen in association with high grade PCa. IDC has been reported to occur in 18 % of radical prostatectomy samples. The incidence is higher in specimens with higher stage. In needle biopsies might be present in about 3 % of cases. Basal cell immunohistochemistry may show complete or partial basal cell layer.
IDC as an isolated finding is rare (<1 % of biopsies) and its clinical significance remains a matter of discussion. An alternative term of atypical cribriform lesion has been suggested to report needle biopsy cases with borderline features between IDC and high grade PIN.
Current approach to IDC is by identifying large acini and ducts containing basal cells filled with malignant epithelial cells that show solid or dense cribriform patterns. In cases with luminal pattern of loose cribriform or micropapillary, marked nuclear atypia (≥6×) or non-focal comedonecrosis is required. Other authors suggest to combine a set of major and minor criteria, as follows:
Major criteria for diagnosis of IDC are:
1.
Large glands (>2× normal)
2.
Presence of basal cells (confirmed by immunohistochemistry)
3.
Cytologically malignant cells with frequent mitosis
4.
Cells spanning gland lumen
5.
Comedonecrosis
Minor criteria for diagnosis of IDC are:
1.
Right-angle branching
2.
Round smooth gland contour
3.
Frequently 2 population of cells
3.4.10 Small Cell Carcinoma
Small-cell carcinomas of the prostate histologically are identical to small-cell carcinomas of the lung and similar diagnostic criteria apply. About 50 % of the patients have mixed small-cell carcinoma and adenocarcinoma of the prostate.
The average survival of patients with small-cell carcinoma of the prostate is less than a year. There is no difference in prognosis between patients with pure small-cell carcinomas and those with mixed glandular and small-cell carcinomas. The appearance of a small-cell component within the course of adenocarcinoma of the prostate indicates a terminal phase of the disease.
Using immunohistochemistry small-cell components are negative for PSA and PAP but positive for neuroendocrine markers and ERG. By FISH analysis, small cell carcinoma from prostate may be differentiated from small cell carcinoma of the bladder since the former retains ERG–TMPRSS2 molecular alterations.
There are conflicting studies as to whether small cell carcinoma of the prostates is positive for thyroid transcription factor-1 (TTF-1), in order to distinguish them from a metastasis from the lung.
Small cell carcinoma of the prostate needs to be differentiated from Gleason pattern 5 which is negative for neuroendocrine markers. No Gleason grade applies to small cell carcinoma (Figs. 3.65, 3.66 and 3.67).
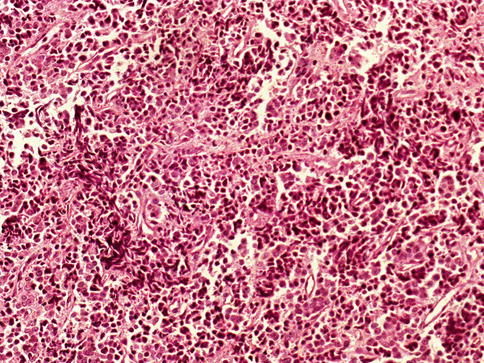
Fig. 3.65
Microscopic appearance of small cell carcinoma of the prostate
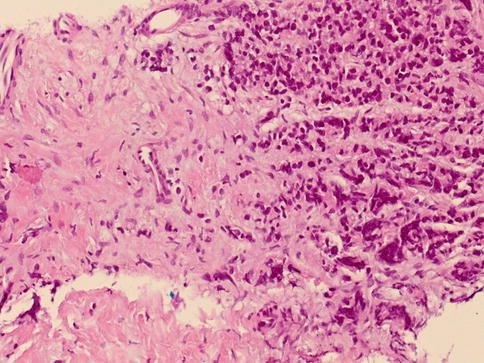
Fig. 3.66
Small cell carcinoma needs to be differentiated from small cell pattern of Gleason 5 prostate adenocarcinoma depicted in this figure
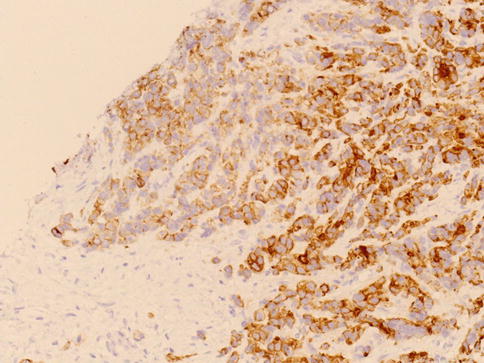
Fig. 3.67
Racemase expression in small cell pattern of Gleason 5 prostate adenocarcinoma
3.4.11 Sarcomatoid Carcinoma (Carcinosarcoma)
In some series, carcinosarcoma and sarcomatoid carcinoma are considered as separate entities based on the presence of specific mesenchymal elements in the former. However, given their otherwise similar clinico-pathologic features and identically poor prognosis, these two lesions are best considered as one entity.
Sarcomatoid carcinoma of the prostate is a rare neoplasm composed of both malignant epithelial and malignant spindle-cell and/or mesenchymal elements. Sarcomatoid carcinoma may be present in the initial pathologic material (synchronous presentation) or there may be a previous history of adenocarcinoma treated by radiation and/or hormonal therapy (metachronous). The gross appearance may resemble sarcoma.
Serum PSA is within normal limits in most cases. Nodal and distant organ metastases at diagnosis are common with less than a 35 % 5-year survival.
Histology
Microscopically, sarcomatoid carcinoma is composed of a glandular component showing variable Gleason score. The sarcomatoid component often consists of a non-specific malignant spindle-cell proliferation (Figs. 3.68 and 3.69).
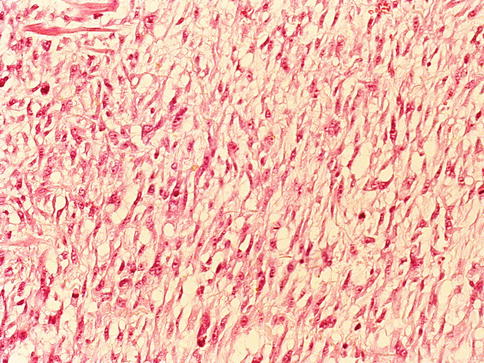
Fig. 3.68
Sarcomatoid carcinoma (carcinosarcoma) of the prostate showing myxoid spindle cell proliferation with high mitotic index
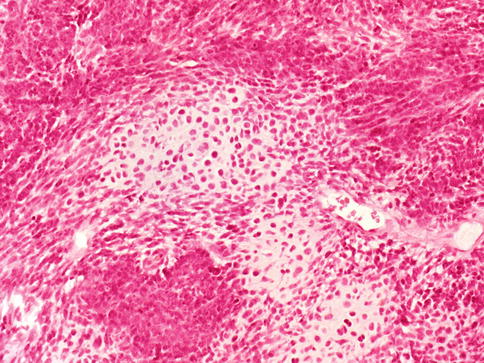
Fig. 3.69
Sarcomatoid carcinoma (carcinosarcoma) of the prostate showing chondrosarcoma and undifferentiated sarcoma
Amongst the specific mesenchymal elements is osteosarcoma, chondrosarcoma, rhabdomyosarcoma, leiomyosarcoma, liposarcoma, angiosarcoma or multiple types of heterologous differentiation. Sarcomatoid carcinoma should be distinguished from the rare carcinoma with metaplastic, benign-appearing bone or cartilage in the stroma.
Epithelial elements react with antibodies against PSA and/or pan-cytokeratins, whereas spindle-cell elements react with markers of soft tissue tumours and variably express cytokeratins or CD10.
3.4.12 PIN-Like Carcinoma
The malignant glands exhibit cellular stratification and thus morphologically resemble PIN. Patients mean age is 68 years. The malignant glands have lumina that may be tufted, micropapillary, flat or a mixture of patterns.
Predominance of stratified tall columnar is similar to ductal carcinoma and therefore it is referred to as PIN-like ductal carcinoma. It is suggested that may behave similar to Gleason score 3 + 3 = 6 cancers.
This unusual form of prostatic carcinoma needs to be distinguished from high grade PIN. Lack of staining for basal cell markers is a diagnostic clue (Figs. 3.70 and 3.71).
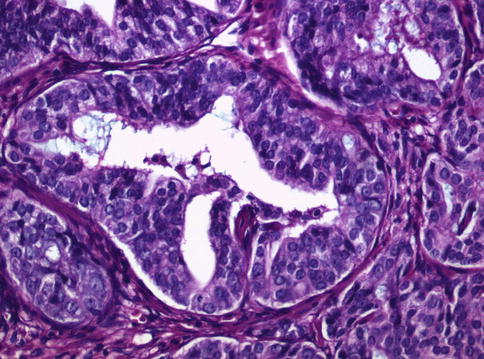
Fig. 3.70
PIN-like subtype of prostate adenocarcinoma
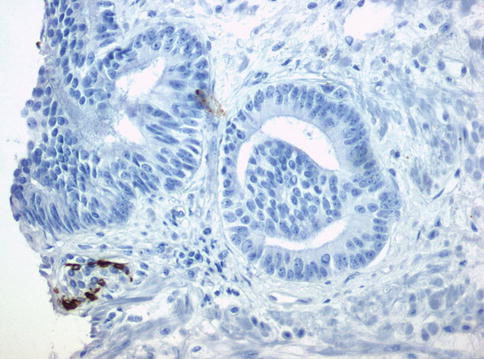
Fig. 3.71
PIN-like subtype of prostate adenocarcinoma. Lack of basal cells as seen by immunohistochemistry with anti-basal cell cytokeratin
3.4.13 Pleomorphic Giant Cell Carcinoma
Extremely rare and aggressive variant of prostate cancer with large bizarre anaplastic giant cells. Mean age at diagnosis is 66 years. Occurs admixed with high grade prostate carcinoma Gleason score 9. Pleomorphic giant cells are DNA aneuploidy and immunoreactive for epithelial markers and focally with PSA and PAP (Figs. 3.72 and 3.73; Tables 3.5 and 3.6).
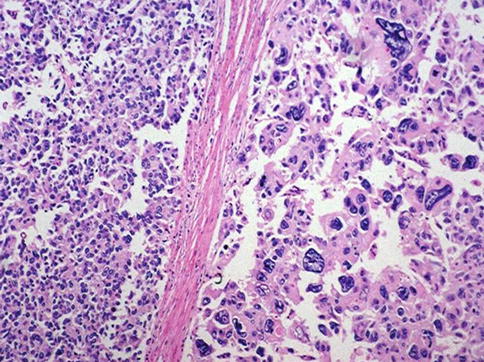
Fig. 3.72
Pleomorphic giant cell carcinoma of the prostate (right) as compared with high grade acinar carcinoma (left)
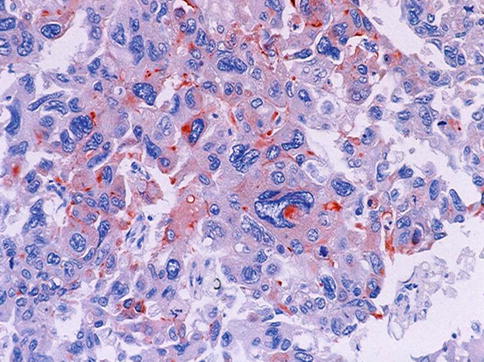
Fig. 3.73
PSA staining of pleomorphic giant cell carcinoma cells
Table 3.5
Gleason grades in histological variants and variations of prostate cancer
Variants
Ductal carcinoma [pattern 4]
Small cell carcinoma [no grade applies]
Mucinous (colloid) carcinoma [pattern 4 (some pattern 3) vs. grade according to underlying pattern]
Mucinous signet ring cell carcinoma [no grade applies]
Sarcomatoid carcinoma [no grade applies]
Pleomorphic giant cell [pattern 5]
Adenosquamous carcinoma [no grade applies]
Lymphoepithelioma-like [no grade applies]
Basal cell/adenoid cystic carcinoma [no grade applies]
Urothelial carcinoma (involving prostatic ducts and acini with or without stromal invasion) [no grade applies]
Squamous cell carcinoma [no grade applies]
Variations
Atrophic features[underlying glandular pattern]
Pseudohyperplastic [pattern 3]
Glomeruloid [pattern 3 or pattern 4]
Collagenous micronodules (mucinous fibroplasia) [underlying glandular pattern (most pattern 3)]
Focal acellular mucin extravasation [ignore mucin and use underlying glandular pattern]
Foamy gland carcinoma [underlying glandular pattern (most pattern 3)]
Non-mucinous signet ring cell features (cytoplasmic vacuoles) [underlying glandular pattern (most pattern 4 or 5)]
Hypernephroid [pattern 4]
Table 3.6
Main clinico-pathological findings in unusual variants of prostate cancer
Clinical features
PSA
IHC
Age at
Incidence
Treatment
Small cellcarcinomaa
Bladder outlet obstruction; disseminated disease; paraneoplastic syndrome
Usually elevated
Cells are positive for at least one of the neuroendocrine marker, i.e. chromogranin A, synaptophysin, neuron specific enolase, CD56 or TTF-1
65–72 years (range 24–89)
0.3–1 %
Chemotherapy
Ductaladenocarcinoma
Obstruction and hematuria are common; some with metastases and advanced clinical stage at diagnosis
Most above 4 ng/ml
PSA and PSAP are almost always positive; Alpha-methylacyl CoA racemase can be detected at a reduced level
63–72 years (range of 41–89 years)
1.3 %b
Androgen deprivation therapy may provide palliative relief, even though this cancer is less hormonally responsive than acinar adenocarcinoma
Sarcomatoidcarcinoma (carcinosarcoma)
Urinary tract obstruction and symptoms of frequency, urgency and nocturia
Variable normal or elevated
Epithelial markers (cytokeratins, PSA, PSAP) and muscle markers can be detected Vimentin immunostains are uniformly positive, and S-100 protein is consistently found in chondrosarcomatous regions, others +
70 years of age (range 50–89)
Rare
Surgery followed by Chemotherapy
Basal cell carcinoma
Urinary obstruction
Variable
stained with 34betaE12 and p63 and negative for PSA, PSAP and alpha-methylacyl-coenzyme racemase; others+
Elderly
Rare
Surgery
Squamous cellcarcinoma
Some arise in prostate cancer patients following endocrine therapy or radiotherapy or schistosomiasis
Most with normal serum PSA and PSAP
negative for PSA and PSAP immunostains
Average age of 68
Less than 0.6 %
Surgery
Adenosquamous carcinoma
50 % may arise in prostate cancer patients following endocrine therapy or radiotherapy
Most with Most with normal serum PSA and PSAP
Negative for PSA and PSAP immunostains
Average age of 68
Rare
Surgery
Primary urothelial carcinoma
Urinary obstruction and hematuria
Variable (limited information)
They are PSA and PSAP negative, and are frequently positive for the cytokeratins CK20, CK7, high molecular weight cytokeratins bound by antibody 34betaE12, p63, thrombomodulin, and uroplakins
(Range 45–90 years)
0.7–2.8 %
Surgery
Pleomorphicgiant cell carcinoma
Urinary obstruction; metastases
Variable (limited information)
PSA, PSAP, Cytokeratins
45–77 years
Very rare
Surgery, androgen blockade, radiation therapy
LELC
Urinary obstruction
5.5 ng/ml
Cytockeratins,PSA and PSAP
66 years
Vary rare
Surgery
3.5 Gleason Grading of Prostate Cancer
The Gleason grading system for prostate cancer is the predominant grading system for prostate cancer around the world. The Gleason grading system is based on glandular architecture which can be divided into five patterns of growth (also known as grades) with decreasing differentiation (Table 3.7).
Table 3.7
Diagnostic reporting of Gleason score
General features
Perform Gleason grading at low magnification using a 4× or 10× lens
Report primary pattern and secondary pattern and assign Gleason score
If only one pattern is present, double it to yield Gleason score. A Gleason score is usually assignable even if the cancer is extremely small
In a needle biopsy with more than 2 patterns (tertiary pattern), the worst pattern must be reflected in the Gleason score even if it is not the predominant or secondary pattern, use the rule “the most and the worst” (see text)< div class='tao-gold-member'>Only gold members can continue reading. Log In or Register to continue
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree

 Get Clinical Tree app for offline access
Get Clinical Tree app for offline access






