The Nonneoplastic Esophagus
Esophageal Development
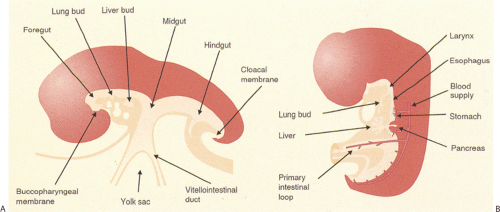
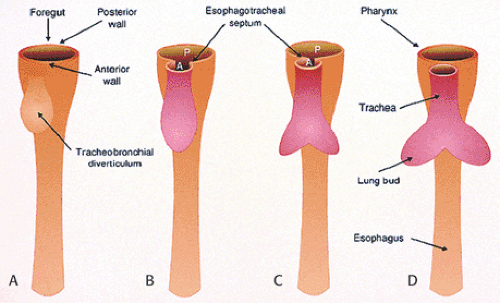
The esophagus develops from the cranial part of the primitive foregut, becoming recognizable at the 2.5-mm stage of development (approximately the third gestational week) as an annular constriction located between the stomach and pharynx (Fig. 2.1) (1). It elongates and grows in a cephalad direction becoming increasingly tubular. Early, the cephalad parts of both the esophagus and trachea lie within a single common tube. Lateral ridges of proliferating epithelium develop in the uppermost segment, dividing the lumen into anterior and posterior compartments. Primitive mesenchyme grows into the forming septum, eventually separating the esophagus and trachea. As soon as the esophagus and trachea divide, the esophagus lies dorsally, and the trachea and lung buds lie ventrally (Figs. 2.1 and 2.2).
The earliest identifiable esophagus consists of two to three layers of pseudostratified columnar cells (Fig. 2.3). The cell layers thicken and then vacuolate (Fig 2.4). Eventually the vacuoles disappear. Abnormalities in the vacuolation process account for the formation of some esophageal cysts. Mucin-secreting cells replace the ciliated cells (2). Glycogenated, nonkeratinized, stratified squamous cells then replace the mucinous epithelium. Squamous cells first appear in the midesophagus and extend both proximally and distally to line the remainder of the esophagus by the fifth gestational month. Submucosal glands appear following the development of the squamous epithelium; they fully mature after birth (2). The implication of these developmental changes is that residual nests of embryonic types of esophageal epithelium may persist in the adult esophagus giving rise to some congenital abnormalities.
Gross Anatomic Features
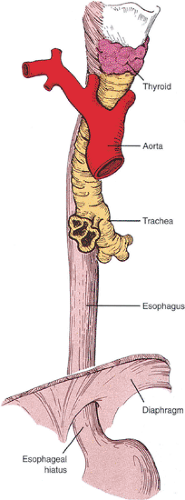
The esophagus begins in the pharynx at the cricopharyngeus muscle and ends at the gastroesophageal junction (GEJ) to the left of midline opposite the 10th or 11th thoracic vertebrae. The esophagus usually measures 25 to 35 cm in adults. For the endoscopist, the esophagus starts 15 cm from the incisor teeth; it ends with the appearance of gastric folds at the GEJ or Z line (Fig. 2.5). The esophagus follows the course of the vertebral column maintaining close proximity with the trachea, left mainstem bronchus, aortic arch, descending aorta, and left atrium (Fig. 2.6). It is customarily divided into thirds. The normal esophagus has constrictions (Fig. 2.7) at its cricoid origin, along its left side at the aortic arch, at the crossing of the left mainstem bronchus, at the fifth thoracic vertebra and left atrium, and where it passes through the diaphragm. These constrictions become clinically significant when food or pills lodge in them making them susceptible to ulceration. The esophagus enters the abdomen, passing through the esophageal hiatus formed by the diaphragmatic muscles. Its intra-abdominal portion measures 1.5 cm in length. The right side of the GEJ appears smooth, whereas the left side forms a sharp angle known as the incisura or angle of His.
Sphincters that maintain esophageal closure under resting conditions lie at the proximal and distal ends. The esophagus is mobile between the upper sphincter and its passage through the diaphragm, allowing it to be displaced by mediastinal or pulmonary diseases. Lower esophageal sphincter (LES) pressure involves a balance between neurogenic and tonic contraction of the musculature and various neural and possibly endocrine and paracrine influences that inhibit contraction resulting in relaxation. The LES keeps the esophageal lumen closed, preventing reflux during rest and regulating food passage into the stomach. The most distal portion of the LES defines the GEJ. The esophageal mucosa has a smooth, featureless, glistening, pink-tan appearance. The squamocolumnar junction appears as a serrated line known as the Z line (Fig. 2.5). Grossly, the Z line consists of small projections of red glandular epithelium measuring up to 5 mm in length and 3 mm in width extending through the pink-white squamous epithelium.
Four arterial groups regionally supply the esophagus: (a) the thyroidal arterial trunk and branches of the subclavian artery that supply the upper esophagus, (b) bronchial arteries and esophageal arch arteries from the upper descending aorta that supply the upper and midesophagus, (c) intercostal arteries and periesophageal arteries from the lower thoracic aorta that supply the distal esophagus, and (d) branches from the inferior phrenic, left gastric, and short gastric artery that supply the diaphragmatic part of the esophagus. The arteries run within the muscularis propria,
giving rise to branches that course through the submucosal plexus (3). Extensive anastomoses among these arterial supplies account for the rarity of esophageal infarction.
giving rise to branches that course through the submucosal plexus (3). Extensive anastomoses among these arterial supplies account for the rarity of esophageal infarction.
Venous drainage also demonstrates a regional distribution. Esophageal veins form a well-developed submucosal plexus that drains into the thyroid, azygos, hemizygous, and left gastric veins, thereby providing links between the systemic and portal venous circulations. The lower esophagus drains into the systemic circulation through branches of the azygos and left inferior phrenic veins. The lower segment also drains into the portal system through the left gastric vein and into the splenic vein through the short gastric veins. The azygos veins ascend on either side of the thoracic segment and drain the midesophagus. The anterior and posterior hypopharyngeal plexus, the superior laryngeal and internal jugular veins, and the inferior thyroidal vein and intercostal veins drain the proximal esophagus. These eventually drain into the superior vena cava.
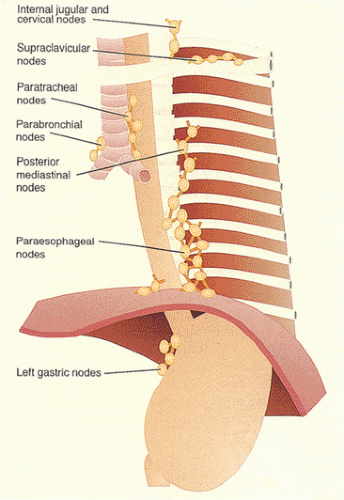
Seven lymph node groups drain the esophagus (Fig. 2.8). The nodes adjacent to the esophagus include paratracheal, parabronchial, paraesophageal, pericardial, and posterior mediastinal lymph nodes. The superior and inferior deep cervical lymph nodes lie further away from the esophagus. In general, the cervical esophagus drains into the internal jugular and upper tracheal lymph nodes. The thoracic esophagus drains into the superior, middle, and lower mediastinal lymph nodes. It also drains into the bronchial and posterior mediastinal paraesophageal lymph nodes and then to the thoracic duct. The distal esophagus drains
into the pericardial lymph nodes at the GEJ. The infradiaphragmatic portion drains to the left gastric and perigastric nodes (4). The two sets of intramural lymphatics lie in the submucosa and in the muscularis propria. The rich mucosal lymphatic plexus connects with a less extensive submucosal one and communicates with longitudinally oriented channels in the muscularis propria. Because of this arrangement, esophageal cancers tend to display early and extensive intramucosal and submucosal intralymphatic spread (see Chapter 3).
into the pericardial lymph nodes at the GEJ. The infradiaphragmatic portion drains to the left gastric and perigastric nodes (4). The two sets of intramural lymphatics lie in the submucosa and in the muscularis propria. The rich mucosal lymphatic plexus connects with a less extensive submucosal one and communicates with longitudinally oriented channels in the muscularis propria. Because of this arrangement, esophageal cancers tend to display early and extensive intramucosal and submucosal intralymphatic spread (see Chapter 3).
Parasympathetic and sympathetic nerves innervate the esophageal mucosa, glands, blood vessels, and musculature. Adrenergic, cholinergic, and peptidergic nerves richly supply the esophageal smooth muscle and serve several neurotransmitter functions, particularly in the LES (5,6,7). LES function is regulated in part by neural nitric oxide synthetase (8). Nitric oxide is a major mediator of LES relaxation. It also initiates the release of and enhances the effect of other transmitters. Intramuscular interstitial cells of Cajal also play an important role in nitric oxide–dependent neurotransmission in the LES (9).
Mucosal Defenses
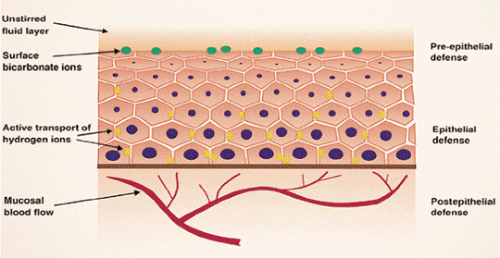
Pre-epithelial, epithelial, and submucosal defenses protect the esophagus from injury. Pre-epithelial defenses include the coordinated actions of the LES and the esophageal muscles to minimize reflux of gastric contents and promote clearance of refluxed material. Microridges on squamous cells hold mucus on their surfaces, providing a protective coat (10). The esophageal epithelium is also protected by a luminal mucus–bicarbonate barrier and hydrophobic surfactants that derive from submucosal and salivary gland secretions. Other salivary components, including mucin, nonmucin proteins, epidermal growth factor (EGF), prostaglandin E2, and carbonic anhydrase also significantly enhance the pre-epithelial barrier.
Epithelial defenses include the glycocalyx, permeability properties of the cell plasma membrane, cells junctions, and ion transport processes that regulate intracellular pH (11). The multiple layers of squamous cells functionally resist damage from the passage of substances over the epithelial surface (Fig. 2.9). Submucosal defenses mainly involve regulation of blood supply via responses of nerves, mast cells, and the blood vessels themselves.
Histologic Features
The Squamous Mucosa
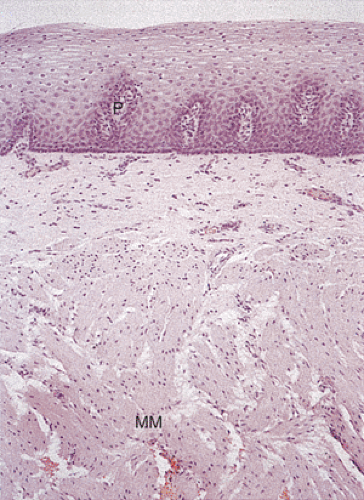
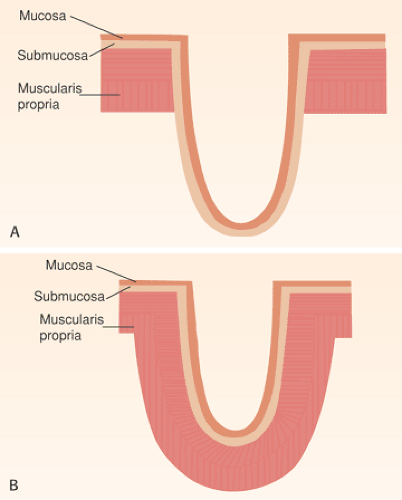
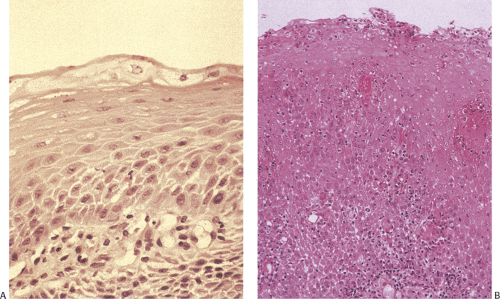
Most of the esophagus is lined by squamous epithelium, except at its distal end. The normal squamocolumnar junction (SCJ) lies at the level of the diaphragm. The squamous mucosa contains three components: Squamous epithelium, lamina propria, and a thick muscularis mucosae (Figs. 2.10 and 2.11). The squamous epithelium consists of nonkeratinizing stratified squamous epithelium (Figs. 2.10, 2.11, and 2.12). The basal zone consists of several layers of cuboidal
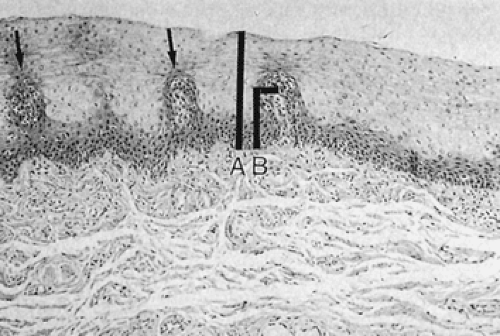
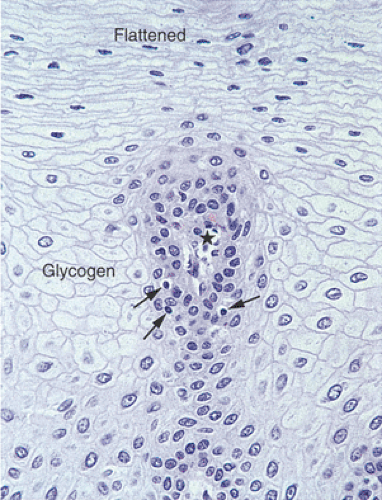
basophilic cells with dark nuclei arranged in an orderly fashion along the basement membrane. Its upper limit is defined as the level where the nuclei are separated by a distance equal to their diameter. It rarely contains mitoses unless some form of injury (esophagitis) is present. The basal layer gives rise to daughter cells that progressively differentiate as they move toward the surface and desquamate. Epithelial cell renewal takes an average of 7 days (12). The basal layer normally occupies the lower 10% to 15% of the epithelium, being one to four cells thick. However, most individuals without evidence of gastroesophageal reflux show basal cell hyperplasia >15% in the distal 3 cm of the esophagus (13). Above the basal cell layer the glycogenated cells progressively flatten as they approach the surface (Fig. 2.13). Periodic acid–Schiff (PAS) stains, which detect intracellular glycogen, facilitate their identification (Fig. 2.14). As one approaches the luminal surface, cell polarity changes from a vertical to a horizontal orientation. This change is accompanied by conversion of a round to an elliptical cell shape. The esophageal mucosa may contain rare keratohyaline granules even though granular and keratinizing layers are usually absent; this finding suggests previous injury.
basophilic cells with dark nuclei arranged in an orderly fashion along the basement membrane. Its upper limit is defined as the level where the nuclei are separated by a distance equal to their diameter. It rarely contains mitoses unless some form of injury (esophagitis) is present. The basal layer gives rise to daughter cells that progressively differentiate as they move toward the surface and desquamate. Epithelial cell renewal takes an average of 7 days (12). The basal layer normally occupies the lower 10% to 15% of the epithelium, being one to four cells thick. However, most individuals without evidence of gastroesophageal reflux show basal cell hyperplasia >15% in the distal 3 cm of the esophagus (13). Above the basal cell layer the glycogenated cells progressively flatten as they approach the surface (Fig. 2.13). Periodic acid–Schiff (PAS) stains, which detect intracellular glycogen, facilitate their identification (Fig. 2.14). As one approaches the luminal surface, cell polarity changes from a vertical to a horizontal orientation. This change is accompanied by conversion of a round to an elliptical cell shape. The esophageal mucosa may contain rare keratohyaline granules even though granular and keratinizing layers are usually absent; this finding suggests previous injury.
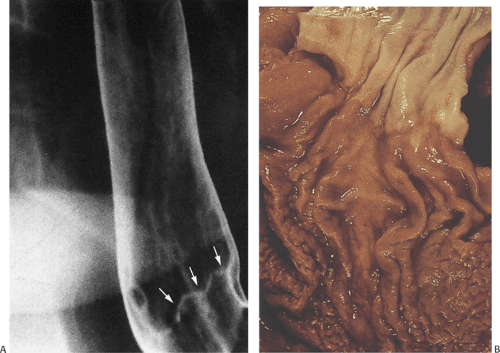
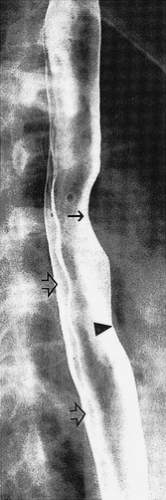 FIG. 2.7. Normal double-contrast esophagogram showing indentation from aorta (arrow), left mainstem bronchus (arrowhead) and indentation of spinal disc spaces (open arrows). |
Endocrine cells lie scattered among the basal cells; they are not present in the mucous glands or ducts. Melanocytes are also present (Fig 2.15) (14). Occasional CD3+ intraepithelial lymphocytes populate the lower and middle squamous cell layers (15). As these lymphocytes interdigitate between the epithelial cells, their nuclei become convoluted, hence the term “squiggle cells.” Antigen-presenting S100+ Langerhans cells lie in a suprabasal location (Fig. 2.16) (15).
Papillae, projections of lamina propria, extend into the squamous epithelium at regular intervals creating an irregular lower border of the squamous epithelium. These papillae normally do not extend more than 50% to 60% through the epithelial height. One measures the height of the papillae from the basal lamina of the surrounding squamous epithelium to the basal lamina at the top of the papilla. The papillae and lamina propria contain blood vessels, lymphatics, fibrovascular tissue, elastic tissue, and occasional inflammatory cells. The lamina propria rests on a two-layer, relatively thick muscularis mucosae. The mucosa is maintained in part by EGF, a mitogenic polypeptide that helps maintain tissue
integrity and cell maturation. The epidermal growth factor receptor (EGFR) possesses tyrosine kinase activity (16) and binds EGF. This may make the esophageal mucosa vulnerable to the anti-EGFR therapies used to treat multiple types of cancer.
integrity and cell maturation. The epidermal growth factor receptor (EGFR) possesses tyrosine kinase activity (16) and binds EGF. This may make the esophageal mucosa vulnerable to the anti-EGFR therapies used to treat multiple types of cancer.
Normal Histology at the Gastroesophageal Junction
The normal histology of the GEJ is a contentious topic. Traditional teaching suggests that the normal Z line is a junction between squamous epithelium and cardiac epithelium with the cardiac mucosa being the most proximal part of the stomach (17). The crux of the current controversy centers on whether the distal esophageal mucosa normally contains cardiac mucosa and whether cardiac mucosa can contain parietal cells. Some suggest that the presence of any parietal cells precludes a histologic diagnosis of cardiac epithelium (18). Others contend that cardiac glands can contain occasional parietal cells provided that other architectural features typical of cardiac mucosa are present (19). Some recommend terms such as oxyntocardiac or cardio-oxyntic or transitional mucosa to describe a cardiac mucosa with occasional parietal cells (18). The histologic controversies are complicated by the fact that there are no uniformly accepted criteria by which the GEJ can be recognized grossly. Thus, it is difficult to establish whether the Z line normally lies precisely at or slightly proximal to the GEJ (20). Furthermore, the upper gastrointestinal (GI) tract easily undergoes metaplastic changes as a result of injury.
Most current authors agree that the extent of the cardiac epithelium is shorter than had been previously suggested. If cardiac epithelium is present at all, it rarely extends more than a few millimeters below the Z line or a few millimeters into the esophagus. Some view the cardia as a normal structure that is present at birth (21,22), whereas others suggest that cardiac mucosa develops as a metaplastic response to gastroesophageal reflux disease (GERD) (23,24,25). Thus, it remains unclear whether there is a tiny band of cardiac mucosa that is a normal structure and whether it lines the esophagus, the proximal stomach, or both. When cardiac mucosa or cardio-oxyntic mucosa overlies submucosal esophageal glands or squamous epithelial-lined ducts, one can be certain that one is in the esophagus and not in the proximal stomach. In the absence
of this landmark, the location is less clear and it is perhaps best referred to as the area of the GEJ. Variability in the extent of the cardiac mucosa likely reflects the presence of underlying disorders such as GERD or Helicobacter pylori gastritis (25) and suggests that the area of the GEJ is a dynamic structure that may change over time.
of this landmark, the location is less clear and it is perhaps best referred to as the area of the GEJ. Variability in the extent of the cardiac mucosa likely reflects the presence of underlying disorders such as GERD or Helicobacter pylori gastritis (25) and suggests that the area of the GEJ is a dynamic structure that may change over time.
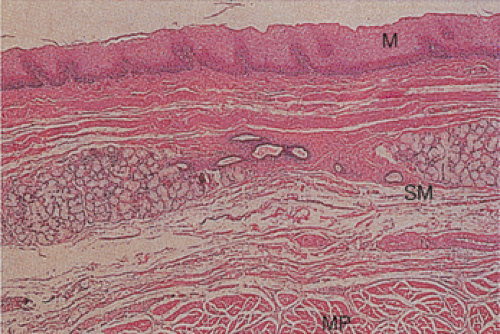 FIG. 2.10. Low-power view of the normal esophagus showing the mucosa (M), submucosa (SM), and muscularis propria (MP). |
In our view, cardiac mucosa consists of a surface mucus-secreting columnar epithelium similar to gastric foveolar epithelium. This epithelium dips down to form foveolae into which branched or compound tubular glands open. In the proximal end of the cardiac mucosa the glands branch freely and show a distinctly lobular architecture (Fig. 2.17). Distally
the glands are less branched and the lobular arrangement becomes less evident. The glands contain mucin-producing cells and may contain parietal cells or even rare chief cells. Abundant endocrine cells are also present. The cells may also blend with pancreatic exocrine cells, a change described in a later section.
the glands are less branched and the lobular arrangement becomes less evident. The glands contain mucin-producing cells and may contain parietal cells or even rare chief cells. Abundant endocrine cells are also present. The cells may also blend with pancreatic exocrine cells, a change described in a later section.
Lamina Propria
The lamina propria constitutes the nonepithelial portion of the mucosa above the muscularis mucosae. It consists of loose areolar connective tissue containing blood vessels, nerves, inflammatory cells, and mucus-secreting glands. Lymphocytes (mostly immunoglobulin-producing B cells), plasma cells, and, occasionally, lymphoid follicles are present.
Muscularis Mucosae
The muscularis mucosae begins at the cricoid cartilage and becomes thicker distally. Proximally it consists of isolated or irregularly arranged muscle bundles rather than
being arranged in a continuous sheet. In the middle and lower esophagus, the muscularis mucosae forms a continuum of longitudinal and transverse fibers that may appear thicker than elsewhere in the GI tract (Fig. 2.11), especially at the GEJ. The muscularis mucosae may appear so thick as to be mistaken for the muscularis propria in biopsy specimens.
being arranged in a continuous sheet. In the middle and lower esophagus, the muscularis mucosae forms a continuum of longitudinal and transverse fibers that may appear thicker than elsewhere in the GI tract (Fig. 2.11), especially at the GEJ. The muscularis mucosae may appear so thick as to be mistaken for the muscularis propria in biopsy specimens.
Submucosa
The submucosa is a wide zone that lies below the muscularis mucosae. It consists of loose connective tissue containing blood vessels, nerves, poorly formed submucosal ganglia, lymphatics, and submucosal glands (Fig. 2.17). The submucosa contains an extensive ramifying lymphatic plexus lying in a loose connective tissue network accounting for early and extensive submucosal spread of esophageal carcinomas. It also contains a rich vascular supply.
There are of two types of submucosal glands: Simple tubular mucous glands called superficial or mucosal mucous glands, and deep or submucosal glands. The former lie in the lamina propria, confined to narrow zones at the distal and proximal ends of the esophagus. They produce neutral mucins and because of their similarity to the glands of the gastric cardia, have also been termed cardiac glands. In contrast, deep or submucosal glands lie in the submucosa, along the length of the esophagus. These glands produce acidic mucins and drain their secretions via ducts lined by columnar epithelium surrounded by myoepithelial cells (26). Submucosal glands contain acini and tubules. From two to four lobules drain into a common duct lined by stratified columnar epithelium that passes obliquely through the muscularis mucosae into the lumen. Loose connective tissue often surrounds these ducts. They vary in position and number from patient to patient and may be purely mucinous, purely serous, or mixed seromucinous in nature. Four types of cells line the submucosal glands: Mucous cells, serous cells, myoepithelial cells, and oncocytes. Mucous cells contain neutral sialated and sulfated mucins. The glands may be surrounded by lymphocytes.
Muscularis Propria
The muscularis propria consists of well-developed circular and longitudinal layers. In its upper part, the muscle fibers assume an oblique orientation and are striated in nature. The striated muscle gradually changes to smooth muscle in the middle third of the esophagus. The LES is not a clearly defined anatomic structure but consists of thickened smooth muscle fibers that extend approximately 2 cm above and 3 cm below the diaphragmatic esophageal hiatus.
Adventitia
The esophagus does not have a serosa as exists elsewhere in the GI tract. Rather, the external part is called the adventitia. It consists of loose connective tissue with longitudinally directed blood and lymph vessels and nerves; it gradually
merges into the loose connective tissue in the mediastinum. Numerous elastic fibers at the GEJ attach the esophagus to the diaphragm.
merges into the loose connective tissue in the mediastinum. Numerous elastic fibers at the GEJ attach the esophagus to the diaphragm.
Cytology of the Normal Esophagus
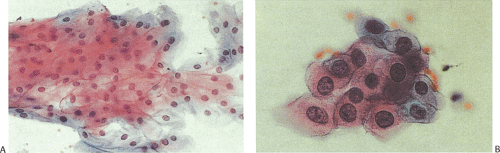
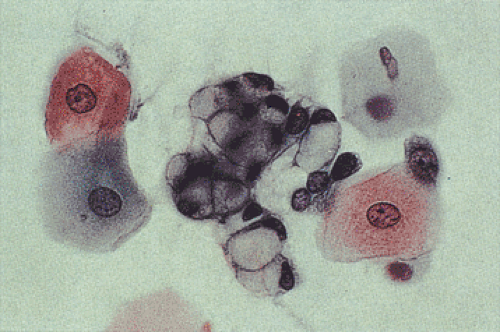
Esophageal cells normally exfoliate from the esophageal mucosa. These include nonkeratinized superficial squamous cells, intermediate cells, and, rarely, parabasal cells (Fig. 2.18). Some squamous cells seen in esophageal cytology specimens derive from the oropharynx. Benign epithelial pearls may occasionally be found. The presence of large numbers of the latter suggests an inflammatory or erosive lesion. Metaplastic squamous cells may exfoliate from the subepithelial mucous glands and their ducts. Benign columnar gastric-type cells (Fig. 2.19) derive from the distal esophagus or from islands of gastric mucosa associated with inlet patches or Barrett esophagus (BE). Foreign material, particularly plant cells, may be present, especially if the esophageal lumen is obstructed. One may also find cells of respiratory origin, such as dust-containing macrophages and ciliated bronchial cells. These usually represent cells that are swallowed, although esophagobronchial or esophagotracheal fistulae or the presence of congenital abnormalities containing bronchial mucosa may account for these cells as well.
Heterotopias
Cervical Inlet Patch
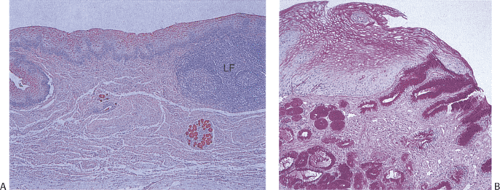
Inlet patches affect 1% to 21% of the population (27,28,29), with the highest incidence occurring during the first year of life. A subsequent decline in incidence suggests that some lesions regress with age. Two pathogenetic mechanisms have been advanced to explain inlet patches. As noted earlier, columnar epithelium lines the fetal esophagus and remnants of columnar or ciliated epithelium may persist leading to the formation of inlet patches. Others suggest that inlet patches represent metaplastic replacement of the squamous mucosa in adults with GERD (30). Those favoring an association with GERD cite similar mucin and cytokeratin patterns in both inlet patches and BE (30). Inlet patches lie in the subcricoid and upper sphincteric region, usually within 3 cm of the upper esophageal sphincter. Most lesions remain asymptomatic. Acid or pepsin secretion by the ectopic gastric mucosa may produce peptic symptoms, ulcers, granulation tissue, webs, strictures, esophagotracheal fistulae, or perforations (31). Adenocarcinomas may also develop, although this is rare (32).
Inlet patches are easily visible velvety, ovoid, pink-red mucosal areas with distinct borders that vary in diameter from a few millimeters to complete esophageal encirclement. Histologically, the lesions consist of cardiac, antral, and/or oxyntic glands covered by foveolar epithelium (Fig. 2.20). In patients with gastric H. pylori (HP) infections, the heterotopic epithelium may become colonized by HP. Intestinal or pancreatic metaplasia may develop (28,29). Large amounts of lymphoid tissue accompany
smaller lesions, as compared to larger ones, suggesting that the lymphocytes play a role in lesional regression. An intense inflammatory reaction may surround the lesion, especially following peptic ulceration.
smaller lesions, as compared to larger ones, suggesting that the lymphocytes play a role in lesional regression. An intense inflammatory reaction may surround the lesion, especially following peptic ulceration.
Heterotopic Gastric Mucosa away from Inlet Patches
Ectopic gastric mucosa also occurs in the mid- or distal esophagus, usually in congenital malformations, including duplications or diverticula. It may also develop following atresia repair (33). Since it often contains oxyntic mucosa, it may present with peptic ulceration.
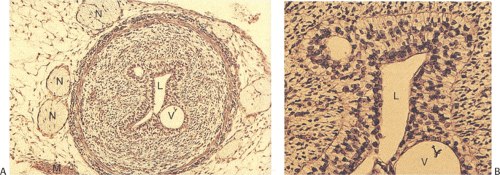
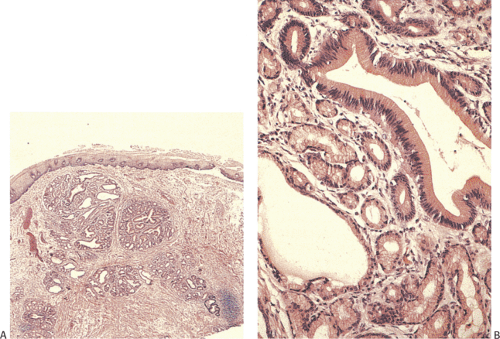
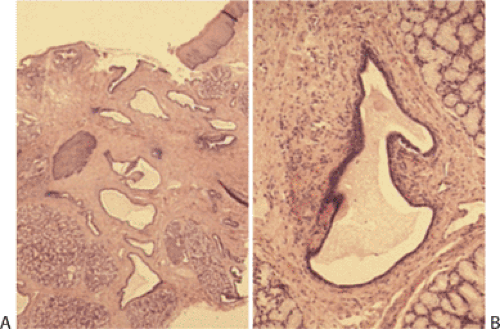
Heterotopic Pancreas
Heterotopic pancreas usually affects the distal esophagus. It may associate with trisomy 18, trisomy 13, esophageal atresia, and esophageal duplication. Complications include fat necrosis, bleeding, ulcers, diverticulum formation, cystic degeneration, inflammation, and, rarely, malignancy. Heterotopic pancreas usually presents as a submucosal mass and contains normal-appearing pancreatic acini and ducts (Figs. 2.21 and 2.22) without islets, although any pancreatic tissue component may be present. Injury due to heterotopic pancreas involves failure of the pancreatic ducts to empty into the esophageal lumen. Eventually the obstructed ducts dilate and rupture, releasing proteolytic enzymes into surrounding tissues. Inflammation and necrosis follow. Heterotopic pancreas differs from pancreatic metaplasia, which is discussed in a later section. The latter is usually a focal change consisting only of pancreatic acini that blend into cardiac
or oxyntic mucosa. Pancreatic ducts are never present in pancreatic metaplasia.
or oxyntic mucosa. Pancreatic ducts are never present in pancreatic metaplasia.
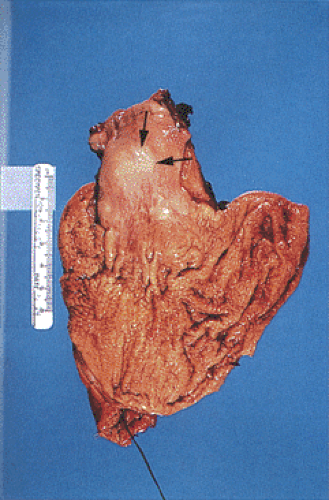 FIG. 2.21. Heterotopic pancreas at gastroesophageal junction presenting as a submucosal mass (arrows). |
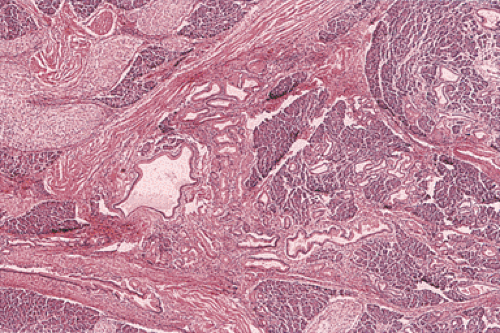 FIG. 2.22. Histologic appearance of the lesion illustrated in Figure 2.21 demonstrating the presence of pancreatic acini and ducts. |
Other Heterotopic Tissues
Ectopic esophageal sebaceous glands present grossly as multiple small, yellowish, mucosal plaques, typically lying in the mid- or distal esophagus. Mature sebaceous glands underlie the squamous mucosa (Fig. 2.23). The lesion represents a developmental abnormality without any clinical significance (34). Patches of ciliated columnar cells may lie within the esophagus. These represent residual fetal remnants and are particularly common in premature infants. They are rarely seen in adults unless they are found in inlet patches, duplications, and/or bronchogenic cysts. Heterotopic thyroid tissue may be present in the esophagus either alone or in tracheobronchial hamartomas.
Developmental Congenital Anomalies
Congenital Esophageal Atresia, Fistula, and Stenosis (Tracheoesophageal Fistula)
Demography and Pathophysiology
Complete esophageal atresia affects 1 in 3,000 live births. Atresia with a common tracheoesophageal fistula occurs in 1 of every 800 to 1,500 live births. Esophageal stenosis affects only 1 in every 25,000 live births (35). Atresia is more common in males than in females. Premature babies and monozygotic twins have a higher risk of atresia than other infants. Occasionally esophageal atresia affects siblings (36). Risk factors include prenatal exposure to lead (37), drugs, and physical agents; maternal diabetes; and a maternal age <20 years (38). Genetic factors include Down syndrome, trisomy 18, and various other chromosomal alterations (39). Severe etiologic insults occur in the first trimester when major organogenesis occurs, often resulting in many associated abnormalities (40).
In esophageal agenesis the proximal primitive foregut develops primarily into a trachea rather than an esophagus (41). Esophageal atresia results from failure of the primitive foregut to recanalize; tracheoesophageal fistula results from failure of the lung bud to separate completely from the foregut. The fistulas that develop vary depending on the amount of epithelium left behind to maintain foregut epithelial continuity. The distal esophageal segment may contain respiratory elements representing a transition zone between the upper foregut, which differentiates into the trachea, and the lower part, which differentiates into the lower esophageal segment (41).
Studies in animal models and in patients with syndromic forms of esophageal atresia indicate the importance of altered genes in the sonic hedgehog signaling pathway. These genes include N-myc and SOX2, which encode transcription factors, and CHD7, which encodes a homeodomain helicase DNA-binding gene, important for chromatin structure and gene expression (42). There are also a number of other genes that are important for normal tracheoesophageal development (Table 2.1); abnormalities in these may also account for some cases of tracheoesophageal malformations.
TABLE 2.1 Genes Necessary for Normal Tracheoesophageal Development | ||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
|
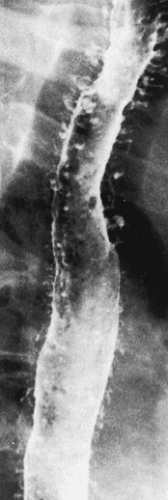
Clinical Features
Esophageal atresia may be detected prenatally by finding a small or absent gastric bubble and maternal polyhydramnios or by the presence of a fluid-filled, blind-ending esophagus (Fig. 2.24). At birth, the presence of a single umbilical artery may alert clinicians to the possibility of esophageal atresia. Infants typically present in the first few hours or days of life with regurgitation, excessive drooling, choking, aspiration, cyanosis, and respiratory distress. Inability to pass a nasogastric tube into the stomach confirms the diagnosis. Air in the stomach and small bowel indicates the presence of a distal tracheoesophageal fistula. Most patients with esophageal stenosis present with dysphagia and regurgitation upon the introduction of solid food.
Approximately one third of infants with esophageal atresia have associated congenital anomalies involving the cardiovascular, gastrointestinal, neurologic, genitourinary, or orthopedic systems (43). Some of the more complex associations are described briefly. The VATER syndrome links vertebral defects, anal atresia, tracheoesophageal fistula, and renal dysplasia (44). The VATER association is defined by finding at least three of the VATER anomalies. A subset of infants with the VATER syndrome have other defects including diaphragmatic, genital, cardiovascular, and neural tube defects; oral clefts; bladder exstrophy; small intestinal atresias; and omphalocele. A variant syndrome, the VACTERL anomaly, combines the VATER syndrome with radial or other limb defects (43). It affects approximately 1.6 of 10,000 live births (45). Defective development of the neural tube and preaxial mesoderm may result in the full spectrum of changes (45). Patients with VACTERL are most likely to be male, have a higher perinatal mortality rate, and have lower mean birth weights than control populations (46). Mothers of infants with VACTERL often exhibit a higher frequency of fetal loss in previous pregnancies than a control population. Patients with a familial form of X-linked VACTERL, X-linked VACTERL-H, develop hydrocephalus due to aqueductal stenosis.
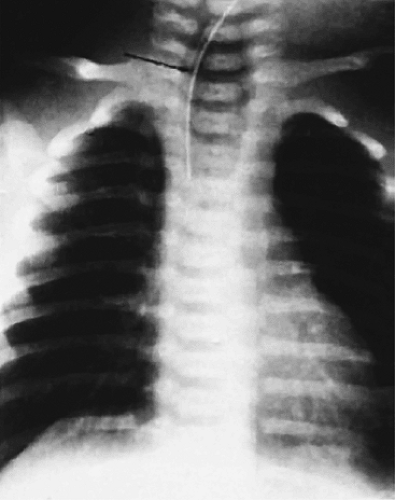 FIG. 2.24. Radiograph of esophageal atresia. The feeding tube (arrow) terminates in the air-distended proximal esophageal pouch. |
Esophageal atresia also associates with the CHARGE syndrome (coloboma, heart disease, atresia choanae, growth and developmental retardation, genital hyperplasia, and ear anomalies) (47). The oculodigitoesophageoduodenal syndrome, also known as Feingold syndrome, is a dominantly inherited combination of hand and foot anomalies, microcephaly, esophageal/duodenal atresia, short palpebral fissures, and learning disabilities. The abnormality maps to chromosome 2p23-p24 (48). The Bartsocas-Papas syndrome consists of bilateral renal agenesis, esophageal atresia, hypoplastic diaphragm, unilateral renal agenesis, agenesis of the penile shaft, or anal atresia (49).
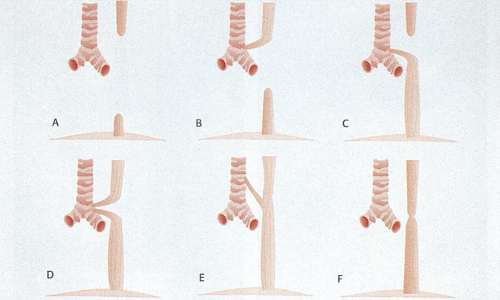
Pathologic Features
Six types of esophageal atresia and stenosis are recognized (Figs. 2.25 and 2.26). In esophageal atresia, hypertrophic esophageal and tracheal muscles intimately blend with one another and the muscle layer contains an extramyenteric plexus. This is a manifestation of incomplete tracheal–esophageal separation. Striated muscle is present in the upper esophagus but not in the lower. Accompanying congenital esophageal neural abnormalities contribute to esophageal dysmotility (50).
Several types of stenosis exist. The stenotic segment varies from 2 to 20 cm in length and is usually located in the mid- or distal esophagus. In the first type of stenosis, segmental narrowing and loss of esophageal mural elasticity produces a localized area of muscular hypertrophy. In rare cases, the muscular hypertrophy involves the entire esophagus. The muscular hypertrophy may result from inflammatory damage to the myenteric plexus, with loss of the muscle-relaxing nitric oxide–producing nerves (51), and may coexist with hypertrophic pyloric stenosis. In a second form of stenosis, cartilaginous tracheobronchial remnants and respiratory epithelium lie within the esophageal wall, as a result of sequestration of a tracheobronchial anlage during the period of cranial elongation before embryologic separation of the esophagus and trachea. In a third form, a membranous diaphragm or web arising from the esophageal wall, containing fibromuscular tissue with a small central perforation, obstructs the lumen. These anomalies are treated surgically but when the lesions are repaired, patients often suffer residual
esophageal dysmotility due to underlying neural abnormalities in the remaining esophagus. The abnormal motility often leads to GERD with all of its complications (52). Restenosis commonly occurs, leading to aspiration and respiratory infections.
esophageal dysmotility due to underlying neural abnormalities in the remaining esophagus. The abnormal motility often leads to GERD with all of its complications (52). Restenosis commonly occurs, leading to aspiration and respiratory infections.
Congenital Bronchoesophageal Fistulas
Bronchopulmonary malformations occur less commonly than tracheoesophageal abnormalities and result from imperfect separation of pulmonary and esophageal anlagen or from an accessory esophageal lung bud. If the communication between the pulmonary tissue and the esophagus is lost, the pulmonary tissue appears as a sequestration. Extralobar sequestrations remain anatomically separate from the lung and have their own pleura. They usually lie adjacent to the esophagus, with which they may communicate. Rarely, intralobar sequestrations communicate with the esophagus presenting as bronchoesophageal fistulas (53). Patients usually present in infancy with a mediastinal mass. Tracheobronchial chondroepithelial hamartomas represent an uncommon related lesion (54), which also results from the abnormal separation of the esophagus and trachea. They contain tracheobronchial lining epithelium, cartilage, and sometimes ectopic thyroid tissue or heterotopic pancreas. These lesions are extremely rare and usually lie in the distal esophagus (54).
Duplications
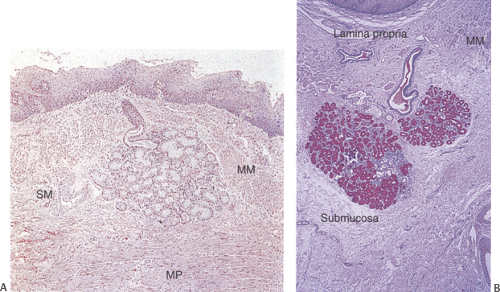
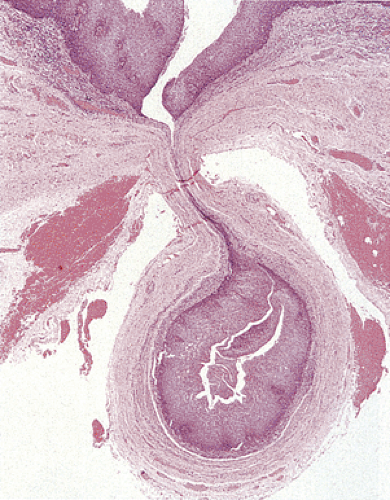
Esophageal duplications account for only 10% to 20% of all GI duplications. They affect approximately 1 of 8,000 persons (55). Duplications develop while columnar, ciliated columnar, or squamous epithelium lines the fetal esophagus and occur in three major forms: Cysts, diverticula (discussed in a later section), and tubular malformations. Cysts account for 80% of duplications. Duplications often present in infancy and childhood with dysphagia, nausea, vomiting, weight loss, pain, bleeding, anorexia, dyspnea, wheezing or recurrent coughing, and pneumonitis.
Duplication cysts are single, fluid-filled cysts (55) that lie posteriorly in a periesophageal location, develop within the esophageal wall (Fig. 2.27), or present as pedunculated intraluminal lesions (56). They average up to 5 cm in diameter. Duplication cysts typically demonstrate continuity of the esophageal muscularis propria with the muscle layer of the cyst wall. It may be difficult to distinguish between esophageal duplication cysts and other intrathoracic cysts
lined by respiratory columnar (Fig. 2.28), cuboidal enteric, stratified squamous, or gastric epithelium. Tubular duplications usually lie within the esophageal wall paralleling the true esophageal lumen. Unlike duplication cysts, tubular duplications communicate with the true lumen at either or both ends of the tube. Duplications usually have a duplicated muscularis propria.
lined by respiratory columnar (Fig. 2.28), cuboidal enteric, stratified squamous, or gastric epithelium. Tubular duplications usually lie within the esophageal wall paralleling the true esophageal lumen. Unlike duplication cysts, tubular duplications communicate with the true lumen at either or both ends of the tube. Duplications usually have a duplicated muscularis propria.
TABLE 2.2 Comparison of Rings and Webs | ||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
|
Bronchogenic Cysts
Bronchogenic cysts lie anteriorly, representing defective tracheoesophageal separation and aberrant bronchial budding from the foregut. They occur in the mediastinum, within the lung, or in the abdomen. Histologically, they contain cartilage, smooth muscle cells, and seromucinous minor salivary glands and are usually lined by ciliated, mucus-secreting, respiratory epithelium. Other bronchogenic cysts are lined by respiratory squamous epithelium but lack cartilage.
Esophageal Rings and Webs
The distal esophagus contains two rings that demarcate the proximal and distal borders of the esophageal vestibule (57). They occur alone or together. The muscular ring lies at its proximal border and corresponds to the upper end of the LES. It is a broad, 4- to 5-mm symmetric band of hypertrophic muscle covered by squamous epithelium that constricts the tubular esophageal lumen at its junction with the vestibule. The mucosal ring or Schatzki ring affects 6% to 14% of individuals and always associates with a hiatal hernia. Mucosal rings are thin, 2-mm transverse mucosal folds that protrude into the esophageal lumen. They usually lie at the SCJ with squamous epithelium covering the upper surface and columnar epithelium lining the lower. The core of the ring contains connective tissue, fibers of the muscularis mucosae, and blood vessels. The muscularis propria contributes little to its formation. Mucosal rings may progress to strictures due to coexisting inflammation (57). Mucosal webs and rings are compared in Table 2.2.
The incidence of esophageal webs ranges from 0.7% to 16%. Congenital esophageal webs are characterized by one or more thin horizontal membranes covered by stratified squamous epithelium arising in the upper and midesophagus. Unlike rings, webs rarely encircle the lumen, but instead protrude from the anterior wall, extending laterally. Webs rarely exceed 2 mm in thickness. Postinflammatory esophageal webs complicate many forms of esophagitis. As a result, they
tend to be multiple and distributed throughout the esophagus. Histologically, webs consist of a thin layer of variably inflamed connective tissue covered on both sides by stratified squamous epithelium. Gastric mucosa may line the undersurface of distal webs. The epithelium covering esophageal webs may undergo neoplastic transformation.
tend to be multiple and distributed throughout the esophagus. Histologically, webs consist of a thin layer of variably inflamed connective tissue covered on both sides by stratified squamous epithelium. Gastric mucosa may line the undersurface of distal webs. The epithelium covering esophageal webs may undergo neoplastic transformation.
The association of cervical webs, dysphagia, and iron deficiency anemia is known as the Plummer Vinson syndrome or Patterson Kelly syndrome (58). It develops in middle-aged women with iron deficiency anemia, glossitis, splenomegaly, and oropharyngeal and esophageal inflammation. Not all patients with the syndrome have webs. Instead, they have nonpropulsive esophageal peristalsis (59) resulting from the iron deficiency, explaining symptom reversal following iron replacement therapy in patients without webs (59). The patients often have other abnormalities, some of which have autoimmune etiologies including autoimmune gastritis, ulcerative colitis, thyroid disease, Sjögren syndrome, and celiac disease (60). Shelflike mucosal webs arise from the anterior wall of the proximal esophagus, occasionally extending laterally or becoming circumferential. They are sometimes multiple. Most lie within 2 to 3 cm of the postcricoid area.
Diffuse Esophageal Intramural Pseudodiverticulosis
Diffuse esophageal intramural pseudodiverticulosis (DEIP) affects 15% to 17% of patients seen at autopsy. Patients range in age from 8 to 83 years (61); males are more commonly affected than females. The disease has several known associations but since many are common, the relationships may be fortuitous rather than etiologic. Possible predisposing factors include alcohol abuse, esophageal reflux, candidiasis, herpes esophagitis, motility disorders, and squamous cell carcinoma. The diverticula result from ductal obstruction caused by inflammation, mucin, or squamous debris. Strictures are present in approximately 75% of patients. Patients present with dysphagia and acute bolus obstruction. These symptoms probably result from coexisting esophagitis or strictures rather than the pseudodiverticula themselves (62).
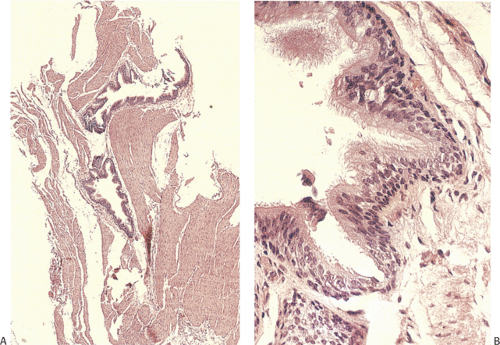
Multiple cystically dilated submucosal glandular ducts produce innumerable 1- to 3-mm flask-shaped diverticula with pinpoint mouths lying evenly distributed in a linear fashion along the esophageal wall (Fig. 2.29). These are most numerous proximally. The intramural cysts extend 3 mm or
less beyond the esophageal lumen. The dilated ducts are lined by stratified squamous epithelium, which may appear hyperplastic (Fig. 2.30). The lumen may contain desquamated squamous cells or inflammatory cells. Organisms, including bacteria, fungi, and parasites, can secondarily colonize the cysts. Nonspecific acute or chronic inflammation often surrounds the acini and the ducts. The inflammation may lead to subsequent submucosal fibrosis or stricture formation.
less beyond the esophageal lumen. The dilated ducts are lined by stratified squamous epithelium, which may appear hyperplastic (Fig. 2.30). The lumen may contain desquamated squamous cells or inflammatory cells. Organisms, including bacteria, fungi, and parasites, can secondarily colonize the cysts. Nonspecific acute or chronic inflammation often surrounds the acini and the ducts. The inflammation may lead to subsequent submucosal fibrosis or stricture formation.
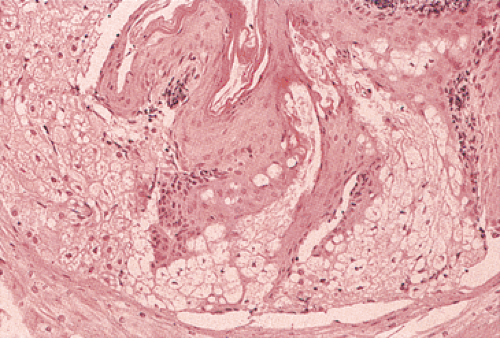
Diverticula
Esophageal diverticula are saccular outpouchings that contain all, or part, of the esophageal wall. One can classify them by their location (pharyngoesophageal, thoracic, or epiphrenic), their pathogenesis (congenital, traction, or pulsion), or their status as true or false, or congenital or acquired (Fig. 2.31). The most important feature distinguishing congenital versus acquired diverticula is the absence of an intact muscularis propria in acquired diverticula. Zenker (hypopharyngeal) diverticulum represents the most common (up to 70%) esophageal diverticulum. Twenty-one percent of diverticula originate in the midesophagus; 8.5% originate in the supradiaphragmatic region. Histologically, squamous epithelium lines all acquired esophageal diverticula unless they develop in an area of Barrett esophagus. Congenital diverticula contain all of the components of the esophageal wall, including the muscularis propria, and may be lined by columnar, ciliated, or squamous epithelium.
Zenker Diverticulum (Pharyngoesophageal Diverticulum)
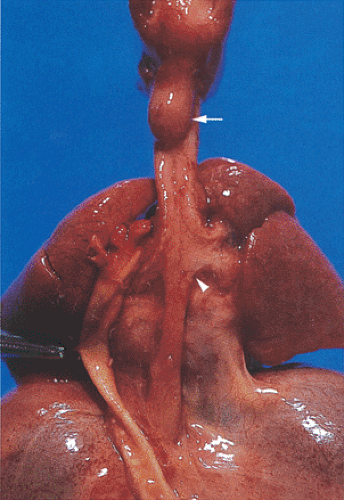
Patients with Zenker diverticula are generally in their 7th or 8th decades of life. There is a 2:1 male preponderance. The diverticulum originates in the proximal esophagus (Fig. 2.32) from mucosal outpouchings at points of weakness in the esophageal wall at its junction with the pharynx. Most develop posteriorly or posterolaterally between the inferior constrictor muscle and fibers of the cricopharyngeus muscle through a triangular zone of sparse musculature termed the Killian triangle. These pulsion diverticula result from uncoordinated muscular contractions during swallowing. As the diverticulum enlarges, it protrudes between the posterior wall of the esophagus and the vertebrae leading to anterior displacement of the proximal esophagus, sometimes causing esophageal compression.
Patients typically present with dysphagia, halitosis, and regurgitation of food consumed several days previously. Aspiration and secondary pneumonitis occur. Secondary bacterial colonization results in diverticulitis. Zenker diverticulum and cervical esophageal webs or hiatal hernias sometimes coexist. Perforation leads to mediastinitis. Squamous epithelium lines Zenker diverticula (Fig. 2.33).
The muscle may appear attenuated and the wall is variably inflamed, sometimes with prominent lymphoid follicles. Ulcers may occur. There is a 0.31% to 0.7% incidence of squamous cell carcinoma secondary to longstanding inflammation (63).
The muscle may appear attenuated and the wall is variably inflamed, sometimes with prominent lymphoid follicles. Ulcers may occur. There is a 0.31% to 0.7% incidence of squamous cell carcinoma secondary to longstanding inflammation (63).
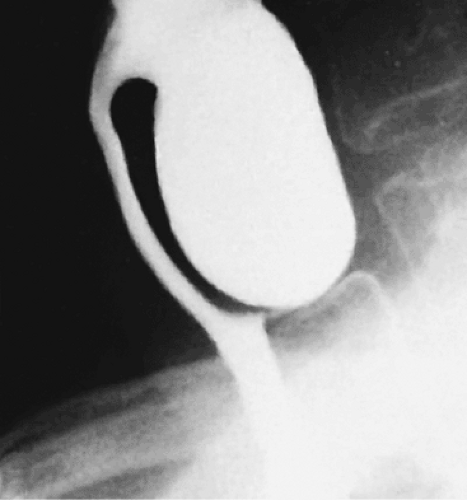 FIG. 2.32. Zenker diverticulum. Lateral view demonstrates the diverticulum as a large saclike structure containing barium. |
Midesophageal Diverticula
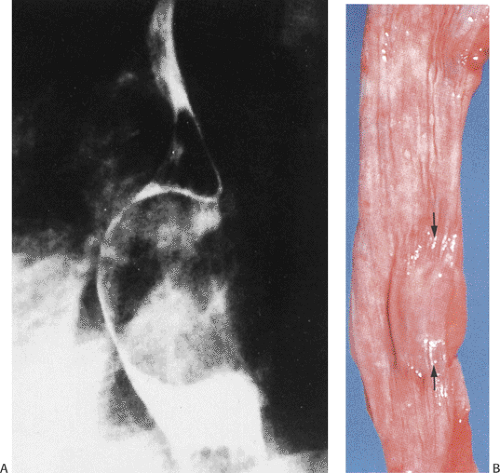
Diverticula arising at the level of the tracheal bifurcation are less common than those in the cervical esophagus. Midesophageal diverticula almost always represent incidentally discovered lesions, unless there is coexisting diverticulitis. They are single or multiple (Fig. 2.34), and usually develop in association with mediastinal inflammation that causes traction on the esophageal wall, pulling it outward. Other diverticula result from motility disturbances, including achalasia or diffuse esophageal spasm. These wide-mouthed diverticula (Fig. 2.35) consist of a variably inflamed squamous mucosa and submucosa with an attenuated muscularis propria.
Epiphrenic Diverticula
Epiphrenic diverticula are rare, developing in middle age, supporting an acquired etiology. They are almost always of the pulsion type, resulting from increased intraesophageal pressure that pushes the mucosa outward in areas of muscular weakness. They frequently coexist with other disorders including hiatal hernia, diaphragmatic eventration, and carcinoma and/or motility disturbances (64). The presence of hypertrophic muscle distal to the diverticula supports the concept that functional or anatomic obstructions are important in their pathogenesis. Patients present with substernal pain, dysphagia, and weight loss. Complications include aspiration pneumonia and lung abscesses, diverticulitis, esophageal obstruction, perforation, mediastinitis, or hemorrhage. Epiphrenic diverticula develop in the distal 10 cm of the esophagus. They appear globular and wide mouthed and, in contrast to midesophageal lesions, may become quite large. The diverticula contain a squamous mucosa and submucosa but no muscularis propria (Fig 2.36). Chronic inflammation is often present. Carcinomas may develop within epiphrenic diverticula for the same reason they do in other diverticula (i.e., stasis of luminal contents), leading to chronic inflammation.
Pancreatic Metaplasia
Pancreatic metaplasia develops in patients with BE (65), carditis, and inlet patches. Mean patient age is 52 years with a range of 18 to 89 years. It occurs in 24% to 60% of patients with biopsy specimens from the SCJ (65,66,67). These glands often lie in the deeper aspects of the mucosa and vary in size from 0.1 to 0.5 mm in greatest diameter (65). They form small clusters of compactly packed cells that either blend imperceptibly into the adjacent metaplastic gastric glands or form distinct nodules that can stand out prominently within
the mucosa. The pyramidal pancreatic acinar cells have abundant apical and midcellular eosinophilic coarse granular cytoplasm and appear basophilic in the basal areas. The basally located nuclei are small, round, and uniform with occasional conspicuous but small nucleoli. The acinar cells are positive for pancreatic lipase and amylase. Mucous cells may intermingle with the pancreatic acinar cells within individual lobules. Endocrine cells may also be present. The foci of pancreatic metaplasia lack pancreatic ducts, periductal smooth muscle fibers, and islet cells.
the mucosa. The pyramidal pancreatic acinar cells have abundant apical and midcellular eosinophilic coarse granular cytoplasm and appear basophilic in the basal areas. The basally located nuclei are small, round, and uniform with occasional conspicuous but small nucleoli. The acinar cells are positive for pancreatic lipase and amylase. Mucous cells may intermingle with the pancreatic acinar cells within individual lobules. Endocrine cells may also be present. The foci of pancreatic metaplasia lack pancreatic ducts, periductal smooth muscle fibers, and islet cells.
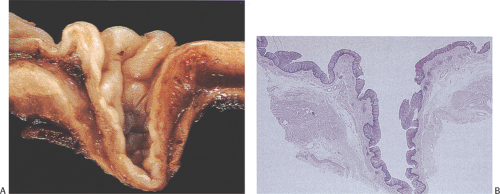 FIG. 2.36. Esophageal diverticulum. A: Midsagittal section of the diverticulum showing lining of tan squamous mucosa; the outer coat is thinned. B: Histologic section of A. |
Mucosal Lacerations, Ulcerations, and Perforations
Esophageal perforation complicates many settings (Fig. 2.37) (Table 2.3). A retrospective study of esophageal perforations in a large hospital found 26 instances in a 10-year time frame. Only six of these were spontaneous, while 19 were due to instrumentation with the largest number due to pneumatic dilation in cases of achalasia (68). Perforations due to foreign bodies occur in areas of physiologic narrowing. Spontaneous transmural perforations qualify for a diagnosis of Boerhaave syndrome. Spontaneous intramural tears qualify for a diagnosis of a Mallory-Weiss tear. When the esophagus perforates, free air enters the mediastinum and spreads to adjacent structures causing palpable cervical emphysema, mediastinal crackling sounds, and pneumothorax. Over time, secondary infections cause mediastinal abscesses, pyopneumothorax, and pleural-pulmonary suppurations.
TABLE 2.3 Causes of Esophageal Perforation | |
|---|---|
|
Mallory-Weiss Syndrome
The term Mallory-Weiss syndrome refers to cases of painless GI bleeding resulting from esophageal or gastroesophageal mucosal lacerations (Fig. 2.37), usually following severe vomiting. Sometimes, vomiting precipitates tears of pre-existing ulcers. Less traumatic events, even snoring, can produce partial esophageal tears, usually above the cardia (69). Most patients are males with a history of alcohol and/or salicylate abuse or hiatal hernias. Rarely lacerations develop in children (70), even neonates. Risk factors for bleeding include portal hypertension or a coagulopathy. These tears account for 5% to 10% of cases of upper GI hemorrhage (71). Single or multiple lacerations lie along the long axis of the distal esophagus crossing the GEJ or
lying in the gastric fundus. Over 75% of lacerations are limited to the stomach; they average 1.5 cm in length. The lacerations vary in depth, often only affecting the mucosa; they rarely extend into the muscularis propria. Submucosal hematomas may form and dissect for a distance beyond the tear. Histologic changes reflect the temporal relationship to the tear. If acute, there may be little in the way of acute inflammation. Over time, acute and then chronic inflammatory cells infiltrate the area around the tear. Previous lacerations may associate with scarring. Bleeding from Mallory-Weiss tears usually stops spontaneously; <5% of patients rebleed.
lying in the gastric fundus. Over 75% of lacerations are limited to the stomach; they average 1.5 cm in length. The lacerations vary in depth, often only affecting the mucosa; they rarely extend into the muscularis propria. Submucosal hematomas may form and dissect for a distance beyond the tear. Histologic changes reflect the temporal relationship to the tear. If acute, there may be little in the way of acute inflammation. Over time, acute and then chronic inflammatory cells infiltrate the area around the tear. Previous lacerations may associate with scarring. Bleeding from Mallory-Weiss tears usually stops spontaneously; <5% of patients rebleed.
Boerhaave Syndrome
The typical patient with Boerhaave syndrome is a middle-aged male, and frequently an alcoholic individual. The disease may also affect children, even neonates (72). Severe vomiting followed by constant excruciating chest pain are the classic clinical signs. Hematemesis occurs at times. The clinical and radiologic findings point to an intrathoracic catastrophe. The nonspecific symptomatology often delays the correct diagnosis. The mortality rate is approximately 31%. The sudden development of a pressure gradient between an internal portion of a viscus and its external supporting tissues represents the common pathogenetic denominator in cases of gastrointestinal rupture (73). The antecedent background varies; the viscus may become overdistended by food, drink, gas, or any combination thereof. Other antecedent events include abdominal blows, straining at stool, parturition, seizures, asthma, prolonged hiccups, and neurologic diseases.
Characteristically, the rent is linear and longitudinal and occurs most commonly in a left lateral posterior location, 1 to 3 cm above the GEJ (Fig. 2.37). The tears measure 1 to 20 cm in length with an average length of 2 cm; the mucosal part of the tear is usually longer than the muscular part. Immediate surgical repair must occur if the patient is to survive. Persistent reflux often follows repair of the rupture (73).
Esophagitis
General Comments
Esophagitis has many causes (Table 2.4), the most common being gastroesophageal reflux, infections, and drugs. Esophageal biopsies are taken to determine the etiology of the esophagitis, to assess the consequences of the inflammation, to follow the course of the underlying disease, and to gauge therapeutic responses. Irrespective of its cause, most cases of esophagitis share common histologic features. Therefore, determining a specific etiology may be difficult unless one detects specific diagnostic features such as the presence of viral inclusions. Additionally, multiple etiologies may be present in any given patient.
Esophageal inflammation can be acute, chronic, or mixed. Mild esophageal injury results in reversible mucosal changes and transient inflammation. Changes associated with acute damage include the presence of balloon cells and inflammatory cells (particularly mononuclear cells) and eosinophils. Basal cell hyperplasia and papillary elongation develop and vascular lakes form. In severe esophagitis, ulcers, erosions, or neutrophils may be seen. Chronic damage leads to submucosal fibrosis or strictures. Patients with longstanding reflux esophagitis may develop Barrett esophagus.
TABLE 2.4 Causes of Esophagitis | |
|---|---|
|
Multinucleated epithelial giant cell changes develop in esophagitis of varying etiologies, representing a nonspecific reparative response. The mucosa contains multinucleated (mean three nuclei per cell, range two to nine) squamous epithelial cells. They are often confined to the basal zone, but sometimes they involve both the basal and superficial epithelium. The nuclei contain single or multiple eosinophilic nucleoli with a perinuclear halo but no inclusions, hyperchromicity, or atypical mitoses (74). Multinucleated cells can also be seen in viral infections, but the use of immunostains or genetic tests allows one to separate nonspecific giant cell changes from virally induced changes.
Cytologic material obtained from patients with esophagitis may show a nonspecific acute and chronic inflammatory infiltrate with mixtures of neutrophils, eosinophils, lymphocytes, plasma cells, histiocytes, and erythrocytes. Epithelial cells, when present, usually appear degenerative.
Reflux Esophagitis
The term gastroesophageal reflux (GER) refers to the retrograde flow of gastric and sometimes duodenal contents into the esophagus. The term gastroesophageal reflux disease is a
symptomatic condition or histopathologic alteration resulting from episodes of GER. Reflux esophagitis describes a subset of GERD patients with histopathologic changes in the esophageal mucosa.
symptomatic condition or histopathologic alteration resulting from episodes of GER. Reflux esophagitis describes a subset of GERD patients with histopathologic changes in the esophageal mucosa.
Demography/Epidemiology
GERD affects patients of all ages, even children and small infants. The prevalence of GERD ranges from 3% to 36%. GERD is equally present among men and women, but there is a male predominance of esophagitis and Barrett esophagus. GERD affects whites more frequently than members of other races. There is also geographic variability in the prevalence of GERD with very low rates in Africa and Asia and high rates in North America and Europe (75). However, GERD is increasing in frequency in Asians. Nonerosive reflux disease appears to be the commonest form of GERD among Asians. Ethnic and geographic demographic differences suggest that both genetic factors and environmental factors play a role in predisposition to GERD (76).
Conditions predisposing to GERD include smoking; increased intra-abdominal or intragastric pressure, including pregnancy, ascites, and obesity; and delayed gastric emptying. Motility disorders including diabetes, alcoholic neuropathies, achalasia, and scleroderma also predispose to GERD. Patients with hiatal hernias and strictures are especially prone to develop GERD. It also follows surgical procedures. Erosive esophagitis is particularly common in acid hypersecretors such as those with the Zollinger-Ellison syndrome (77). GER in infants and children complicates congenital esophageal or gastric abnormalities. GER also associates with cystic fibrosis (78).
Pathophysiology
GERD is a multifactorial disorder (Table 2.5). Most patients have a lower mean LES resting pressure than is seen in patients without GERD. This allows acid to reflux into the esophagus, leading to the development of esophagitis. The inflammation further impairs LES pressure, increasing acid exposure in the esophagus (79). Patients also have inadequate or slowed clearance of refluxed material and delayed gastric emptying and/or increased gastric volume. The nature and amount of refluxed material and the length of time it remains in contact with the esophageal mucosa as well as the number of reflux episodes determine whether GERD develops (80). GERD results from reflux of both acid and alkaline secretions (Fig. 2.38). Acid alone causes relatively few changes, but when combined with pepsin or bile acids, more severe damage results (81). Pepsin requires an acid pH to exert its full damaging effects (82). Patient age, nutritional status, and other less well-understood factors also influence the mucosal capacity to withstand injury and to repair itself following injury. Reflux esophagitis may also be enhanced by the genesis of free radicals during reflux. These free radicals damage cell membranes, thereby altering the mucosal barrier. Lipid peroxidation increases with the increasing grade of esophagitis; it is highest in patients with BE (83).
TABLE 2.5 Causes of, or Predisposition to, Gastric Reflux | |
|---|---|
|
Relationship of Reflux Esophagitis to Helicobacter pylori Infections
Studies addressing the relationship of HP infection to GERD often reach conflicting conclusions. This results from the fact that the interplay of HP infections and GERD is complex and complicated by the common use of proton pump inhibitor (PPI) therapy in these patients. At the heart of the debate is the link between gastric acid secretion, HP infection, and GERD. In patients with gastric ulcer and corpus gastritis, the impact of HP infection varies substantially producing wide variation in patterns of acid secretion. In many patients gastric acid is suppressed and is no longer produced in the
amount necessary to induce GERD. Bacterial eradication in some of these individuals results in a substantial recovery of acid secretion with sufficient acid to increase the aggressiveness of refluxed gastric juice to the esophageal mucosa (84). In contrast, duodenal ulcer patients typically have antrum-predominant HP gastritis and a well-preserved acid-secreting mucosa. In these patients, HP infections may make the acid-secretory mechanism hyperresponsive to stimulation, increasing acid production. In this patient group, HP infections can increase the aggressiveness of the gastric juice to the esophageal mucosa.
amount necessary to induce GERD. Bacterial eradication in some of these individuals results in a substantial recovery of acid secretion with sufficient acid to increase the aggressiveness of refluxed gastric juice to the esophageal mucosa (84). In contrast, duodenal ulcer patients typically have antrum-predominant HP gastritis and a well-preserved acid-secreting mucosa. In these patients, HP infections may make the acid-secretory mechanism hyperresponsive to stimulation, increasing acid production. In this patient group, HP infections can increase the aggressiveness of the gastric juice to the esophageal mucosa.
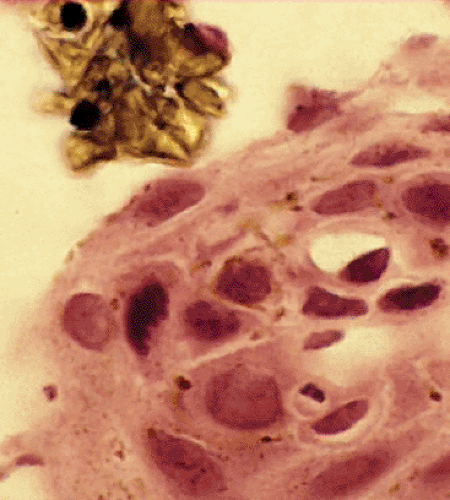 FIG. 2.38. Bile reflux esophagitis. Surface of the esophageal mucosa demonstrating the presence of bile crystals overlying the squamous epithelium. |
Current epidemiologic trends indicate an inverse relationship in the Western world between the rising incidence of GERD and the decreasing incidence of HP infections (85). The lower prevalence of HP in GERD patients, the increase of GERD following HP eradication, and the association with certain gastritis patterns (atrophic corpus gastritis) have led to the widespread opinion that HP exerts a protective effect on the esophagus and may prevent the development of GERD and its complications (85). However, as noted, the data and their interpretations are conflicting, keeping this subject one of continuing interest.
Clinical Features
Transient mild reflux affects most individuals including children and adults. It is especially common in preterm infants (86). The degree of reflux must be severe for individuals to become symptomatic. Fifty percent of symptomatic patients have complications including esophagitis, strictures, or BE. Adults present with diverse symptoms including heartburn, regurgitation, bitter-tasting fluid in the mouth, dysphagia, odynophagia, nausea, vomiting, hiccups, anginalike chest, and hoarseness. The regurgitation can cause a spectrum of conditions, including asthma, chronic laryngitis or pharyngitis, subglottic stenosis, and dental disease. Some patients present with bleeding from esophageal ulcers. Complications peak between ages 50 and 70 years (87). The severest complication is carcinoma developing in the setting of BE.
The clinical course and prognosis of infants and children with GER differ depending on the age at onset. Some children present with symptoms of asthma. GERD may also cause obstructive apnea in infants. Severe dental caries are common. The most frequent complication of recurrent GER in children is failure to thrive as the result of caloric deprivation, vomiting, recurrent bronchitis, or pneumonia caused by repeated episodes of pulmonary aspiration. Some children require gastroesophageal fundoplication and/or pyloroplasty to alleviate the symptoms.
Gross and Endoscopic Features
The gross appearance of the esophagus varies with disease severity. Approximately one third of patients with chronic GERD symptoms are endoscopically normal (88). Low-grade esophagitis is only evident histopathologically. Areas of patchy erythema and red streaks are the first endoscopic abnormalities. Later erosions and ulcers develop; these predominate distally and taper off proximally. The esophagus appears friable, diffusely reddened, and hemorrhagic (Fig. 2.39); it bleeds easily. As the disease progresses, the ulcers become confluent, even circumferential. Strictures or BE characterize severe chronic disease. Prolonged reflux may result in esophageal shortening. Inflammatory polyps may be present. The distinction between the squamous and the columnar epithelium becomes less clear. Several endoscopic classifications have been developed to evaluate the esophageal mucosa. The two most common are the Savary-Miller MUSE system and “Los Angeles” classifications (89,90).
Histologic Features
Biopsies are performed to confirm the diagnosis of GERD; to document complications, including esophagitis, BE, or tumor development; and to rule out the presence of coexisting infections. Since esophagitis tends to be a patchy process, it is easy to miss diagnostic changes on a single biopsy. The current wisdom is that biopsies should be taken in the area just distal to the Z line to detect carditis (see below), just proximal to the Z line to detect esophagitis, and 3 cm proximal to the Z line to detect the hyperplastic changes that are more predictive of the presence of GERD than more distally derived biopsies.
Repetitive episodes of tissue injury and healing produce histologic features that reflect disease activity at the time of
examination, superimposed on changes from previous injurious episodes. Esophagitis can heal completely or it may progress on to a number of the complications discussed later. Biopsies from patients with heartburn commonly show only basal cell hyperplasia without inflammation. The basal hyperplasia can progress to frank esophagitis. There are four stages of reflux esophagitis: (a) acute (necrosis, inflammation, and granulation tissue formation); (b) repair (basal cell hyperplasia and elongation of the papillae); (c) chronic (fibrosis and formation of Barrett esophagus); and (d) complications (dysplasia and adenocarcinoma).
examination, superimposed on changes from previous injurious episodes. Esophagitis can heal completely or it may progress on to a number of the complications discussed later. Biopsies from patients with heartburn commonly show only basal cell hyperplasia without inflammation. The basal hyperplasia can progress to frank esophagitis. There are four stages of reflux esophagitis: (a) acute (necrosis, inflammation, and granulation tissue formation); (b) repair (basal cell hyperplasia and elongation of the papillae); (c) chronic (fibrosis and formation of Barrett esophagus); and (d) complications (dysplasia and adenocarcinoma).
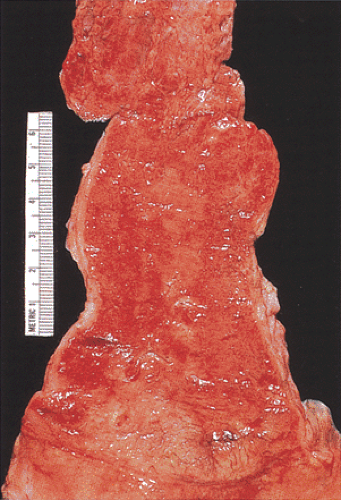 FIG. 2.39. Reflux esophagitis. Area of the Z line is destroyed. Acute hemorrhagic ulcerative reflux esophagitis demonstrating multiple areas of ulceration and erythema. |
Various histologic features should be assessed when examining the biopsy for GERD (Table 2.6). No single feature described below represents an absolute criterion for the presence of GERD, but each is helpful in suspecting the diagnosis. In the absence of a known drug history or the presence of specific microorganisms, biopsies, particularly distal biopsies showing esophagitis, are most likely to be due to GERD.
Epithelial Hyperplasia
The normal basal cell layer is only one to four cells high; it should not constitute more than 15% of the epithelial thickness. In the setting of GERD, the basal zone increases from 10% to more than 50%; papillary height can increase to more than 50% to 75% of the total epithelial thickness (91). This change affects patients with an endoscopically normal mucosa as well as those with endoscopic evidence of esophagitis. Regenerative changes are characterized by nuclear enlargement, hyperchromasia, and mitoses that remain limited to the basal layer (Fig. 2.40). Prominent nucleoli may be present. EGFR expression is enhanced in the hyperplastic cells (92).
TABLE 2.6 Histologic Features of Acute Esophagitis | |
|---|---|
|
Accurate assessment of the basal and papillary height requires evaluating well-oriented specimens. It is helpful to divide the epithelial thickness into thirds. Papillae should not extend into the upper third. When the lower third is divided in half, the basal cells should be confined to its lower half. PAS stains may help distinguish the basal layer from the superficial layers enabling more accurate measurements of basal cell hyperplasia (Fig. 2.41). In less optimally oriented specimens, basal zone thickness can be evaluated if one sees at least three to four papillae arranged in parallel to one another and not cut tangentially. In tangentially cut sections, a helpful feature is an increase in the number of papillae, which can be evaluated in an en face section. In this setting one may see overlapping capillaries. Since the biopsies may be small or have minimal or no lamina propria or they may be inappropriately oriented and therefore difficult to evaluate for basal hyperplasia and papillary elongation, we recommend that the biopsies be examined at three levels to increase their diagnostic accuracy.
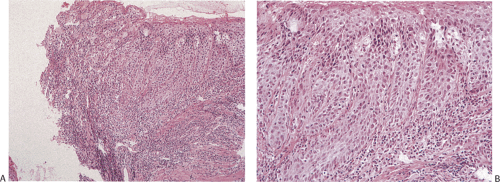
If marked squamous epithelial hyperplasia develops, the elongated epithelial pegs extend into the underlying lamina propria, in a process known as acanthosis (Fig. 2.42). Extensive acanthosis, also termed pseudoepitheliomatous hyperplasia, can suggest the presence of an invasive carcinoma. The epithelium may appear markedly regenerative with cytoplasmic basophilia, an increased nuclear-to-cytoplasmic ratio, glycogen depletion, and increased mitotic activity. However, the reactive cells more or less maintain their polarity and abnormal mitoses are absent. The individual cell keratinization seen in high-grade dysplasia is absent. The cell nuclei may have prominent nucleoli but the nuclei appear relatively uniform in size and one may see some evidence of
squamous cell maturation in the more superficial cell layers. The boundary between the epithelium and the underlying stroma appears smooth, unless extensive inflammation occurs.
squamous cell maturation in the more superficial cell layers. The boundary between the epithelium and the underlying stroma appears smooth, unless extensive inflammation occurs.
 FIG. 2.41. Periodic acid–Schiff stain demonstrating the regularity of the underlying basement membrane and lack of glycogenation of the epithelium. |
The stroma underlying the epithelium may appear inflamed, but one should not see desmoplasia. Very small or poorly oriented biopsies, especially those with significant inflammation and associated inflammatory atypia, may be impossible to interpret. If one is completely unable to distinguish between reactive atypia and dysplasia, a diagnosis of indefinite for dysplasia can be made. In this situation, one may want to recommend repeat biopsies once the reflux has been treated, to rule out the presence of an ulcerated and inflamed carcinoma. The sensitivity of hyperplasia as a diagnostic feature is only 60% to 70% (93). Basal cell hyperplasia is a reversible change that disappears with treatment. Hyperplasia also complicates other forms of esophagitis so that it is not specific for GERD.
Balloon Cells
Balloon cells often develop in the midzone of the epithelium clustering around vascular papillae (94). These occur in approximately two thirds of cases of GERD (Fig. 2.43). The cytoplasm of the enlarged, globoid cells appears swollen; cells contain irregular pyknotic nuclei or demonstrate karyorrhexis.
Balloon cells can be accentuated by PAS stains since they lose the normal intensity of PAS staining characteristic of superficial epithelium. PAS stains also help distinguish balloon cells from the enlarged squamous cells seen in patients with glycogen acanthosis. The presence of balloon cells does not establish a diagnosis of GERD, since they develop in any damaged mucosa, irrespective of its cause. Nonetheless, in the absence of other more characteristic features, the presence of balloon cells may be the only clue to suggest that some form of injury has occurred.
Balloon cells can be accentuated by PAS stains since they lose the normal intensity of PAS staining characteristic of superficial epithelium. PAS stains also help distinguish balloon cells from the enlarged squamous cells seen in patients with glycogen acanthosis. The presence of balloon cells does not establish a diagnosis of GERD, since they develop in any damaged mucosa, irrespective of its cause. Nonetheless, in the absence of other more characteristic features, the presence of balloon cells may be the only clue to suggest that some form of injury has occurred.
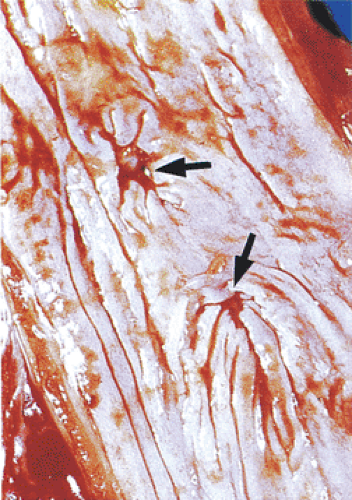
Vascular Changes
Papillary capillary ectasia (sometimes called vascular lakes) (Fig. 2.44) and hemorrhage represent an early but nonspecific histologic sign of GERD. These lakes correspond to the red streaks seen endoscopically. Capillary ectasia develops in up to 83% of patients with reflux, contrasting with its presence in only 10% of control patients (80). This change is often present in the absence of any inflammation. Dilated and congested venules are seen high up at the top of the lengthened papillae. This may be accompanied by mucosal red cell extravasation. This change develops in many other forms of esophagitis.
Inflammation
Lymphocytes.
Small numbers of lymphocytes populate both the normal mucosa and the lamina propria so that their presence does not aid in making a diagnosis of esophagitis. However, they are very conspicuous in patients with GERD (95). Biopsies with esophagitis average greater than six lymphocytes per high-powered field (hpf) (96). Since the lymphocytes have irregular
elongated nuclear contours, they are sometimes referred to as “squiggle cells” or as “cells with irregular nuclear contours.” Squiggle cells contain scant to invisible cytoplasm. The nuclear shape often curves to fit between the squamous cells (Fig. 2.45). Squiggle cells exhibit a T-lymphocyte phenotype (96). They are part of the inflammatory response in GERD but are not an independent marker of reflux esophagitis (95). A small percentage of the lymphocytes are intraepithelial S100+ antigen-presenting cells. Occasionally, the mononuclear cell infiltration becomes severe enough to cause a focal lymphoid hyperplasia mimicking a lymphoma.
elongated nuclear contours, they are sometimes referred to as “squiggle cells” or as “cells with irregular nuclear contours.” Squiggle cells contain scant to invisible cytoplasm. The nuclear shape often curves to fit between the squamous cells (Fig. 2.45). Squiggle cells exhibit a T-lymphocyte phenotype (96). They are part of the inflammatory response in GERD but are not an independent marker of reflux esophagitis (95). A small percentage of the lymphocytes are intraepithelial S100+ antigen-presenting cells. Occasionally, the mononuclear cell infiltration becomes severe enough to cause a focal lymphoid hyperplasia mimicking a lymphoma.
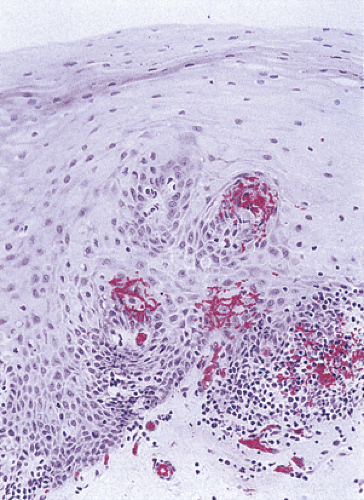 FIG. 2.44. Patients with esophagitis often develop vascular lakes and extravasated red cells. These begin at the area of the papillae and extend outward. |
Neutrophils.
The presence of isolated neutrophils, either in the squamous epithelium or in the lamina propria, serves as evidence for acute esophagitis of many etiologies. Neutrophils are present in the epithelium of 20% or less of patients with reflux esophagitis, making them a relatively insensitive marker. They tend not to appear until the inflammation becomes severe and the epithelium ulcerated. Large collections of neutrophils suggest that a biopsy comes from the area of an ulcer or erosion. Neutrophils decrease in number the further one goes away from the erosion or ulcer. Neutrophils are most commonly detected near the Z line.
Eosinophils.
A modest number of eosinophils (at least six) in the epithelium or in the lamina propria strongly suggest
the diagnosis of GERD (Fig. 2.46) (97). Infiltration by eosinophils in GERD is more common in children than in adults. It occurs early and may occur in the absence of basal cell hyperplasia. Other causes for mucosal eosinophilia include the entities listed in Table 2.7. One can easily appreciate eosinophils in small endoscopic biopsies, even when the biopsies are not well oriented. They affect up to 60% of adults with severe disease but the intraepithelial eosinophils may be focal in nature, necessitating a search for them on serial sections; their presence does not correlate with disease severity (95,97). Eosinophils are not a sensitive marker for GERD, since they are only found in 40% to 50% of individuals with GERD. Significant esophageal eosinophilia (>20 intraepithelial eosinophils per hpf) and eosinophilic microabscesses are not characteristic of GERD but are part of the entity known as eosinophilic esophagitis discussed in a later section.
the diagnosis of GERD (Fig. 2.46) (97). Infiltration by eosinophils in GERD is more common in children than in adults. It occurs early and may occur in the absence of basal cell hyperplasia. Other causes for mucosal eosinophilia include the entities listed in Table 2.7. One can easily appreciate eosinophils in small endoscopic biopsies, even when the biopsies are not well oriented. They affect up to 60% of adults with severe disease but the intraepithelial eosinophils may be focal in nature, necessitating a search for them on serial sections; their presence does not correlate with disease severity (95,97). Eosinophils are not a sensitive marker for GERD, since they are only found in 40% to 50% of individuals with GERD. Significant esophageal eosinophilia (>20 intraepithelial eosinophils per hpf) and eosinophilic microabscesses are not characteristic of GERD but are part of the entity known as eosinophilic esophagitis discussed in a later section.
Erosions, Ulcers, and Fistulas in Gastroesophageal Reflux Disease
The mucosal changes of reflux esophagitis range from the changes already described to acute esophagitis, erosions, superficial ulcers (Figs 2.47, 2.48, and 2.49), and extension of the inflammatory process, leading to fistula formation. The depth of the process distinguishes an esophageal erosion from an ulcer. Erosions are superficial lesions that remain confined to the lamina propria and muscularis mucosae sparing all but the most superficial layers of the submucosa. The necrosis, hemorrhage, and inflammation associated with ulcers extend deeper into the underlying submucosa or muscularis propria. The epithelium close to erosions or ulcers often contains neutrophils, eosinophils, and many lymphocytes. The erosions or ulcers (Fig. 2.49) often contain granulation tissue, an inflammatory exudate, and fibrinoid necrosis in the ulcer base. Lymphoplasmacytic infiltrates, often forming lymphoid aggregates, tend to cluster around erosions and ulcers. Epithelium at the ulcer margin is usually attenuated. Marked basal cell hyperplasia may occupy the entire mucosal thickness and there may be marked acanthosis. These changes may be accompanied by occasional bizarre epithelial or stromal cells.
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree