The Neoplastic Esophagus
Esophageal tumors arise in any of the tissues comprising its four layers: The mucosa, submucosa, muscularis propria, and adventitia. Many types of carcinoma arise in the esophagus, but >70% to 95% are either squamous cell carcinomas (SCCs) and their variants or gastroesophageal junction (GEJ) adenocarcinomas arising in the setting of Barrett esophagus (BE). This chapter focuses on epithelial tumors. The World Health Organization (WHO) classification of epithelial tumors is shown in Table 3.1 (1). WHO staging for esophageal carcinomas is shown in Table 3.2. Mesenchymal tumors are discussed in Chapter 19. Hematologic tumors are discussed in Chapter 18. Neuroendocrine tumors are discussed in Chapter 17. Esophageal epithelial neoplasms are more likely to be malignant than benign. Esophageal squamous carcinomas are the eighth most common cancer worldwide and the sixth most common cause of cancer-related deaths (2). Adenocarcinomas at and proximal to the GEJ are less common, but are increasing in frequency in North America (3) and Europe (4).
Benign Squamous Papillomas
Squamous papillomas are benign exophytic esophageal tumors that fall into two major types: Those associated with human papillomavirus (HPV) infection, often referred to as condylomas, and those unassociated with HPV, usually referred to as squamous papillomas. Their prevalence ranges from 0.01% to 1% (5,6). Squamous papillomas may arise on a background of esophagitis due to reflux or other causes. Some result from the synergistic action of mucosal irritation (as from gastric acid reflux) and HPV infection (7). Evidence of HPV infection is most likely when the papillomas demonstrate a condylomatous histology (5,8,9). In this setting, HPV 16 is most commonly present followed by HPV types 18, 6b, and 11.
Esophageal squamous papillomas may be asymptomatic or present with dysphagia, heartburn, or hematemesis. Patients range in age from 2 to 86 years, with a male:female ratio of 24:9. Esophageal papillomatosis affects children with laryngeal papillomatosis, and is usually related to HPV infection (10). HPV-related esophageal papillomas are rare in adults. Acanthosis nigricans, a rare hereditary condition, associates with multiple esophageal papillomas and late-onset hyperkeratosis (tylosis) of the palms and soles.
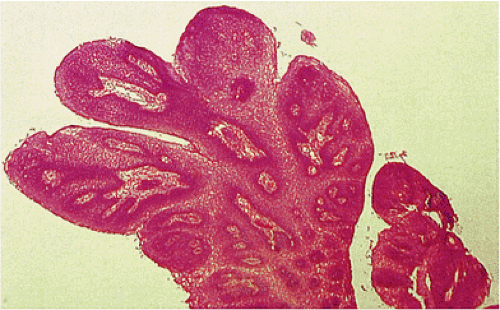
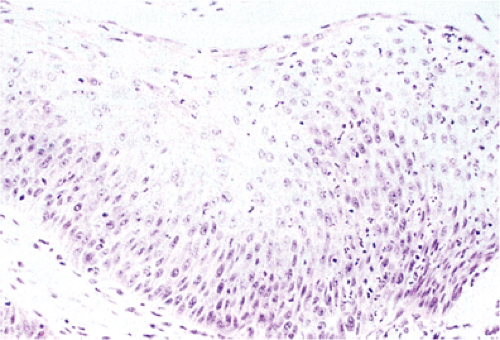
Squamous papillomas usually arise in the lower esophagus where they are generally single, exophytic, multilobulated, soft, semipedunculated, pinkish-white lesions with smooth or slightly rough surfaces. They measure from 0.2 to 1 cm in diameter with an average size of 0.4 to 0.5 cm (5,11). Some patients have multiple lesions, numbering from 2 to more than 20 (5). Benign, proliferating, hyperplastic (thickened and acanthotic) stratified squamous epithelium covers inconspicuous connective tissue cores containing stromal cells and thin-walled vessels (Fig. 3.1). The squamous cells demonstrate orderly cellular maturation from the basal layer toward the surface (Fig. 3.2). The basal cells may appear prominent but they lack significant cytologic atypia. The squamous epithelium of distal papillomas may exhibit the characteristic features of reflux esophagitis.
Esophageal papillomas demonstrate several distinct architectural patterns (5). Exophytic lesions have smooth, fingerlike, papillary, and acuminate configurations with central fibrovascular cores extending to the surface of the papillae (Fig. 3.1). Endophytic lesions consist of benign squamous cell proliferations with an inward or papillomatous proliferation of the surface epithelium. The spiked type has a spiked surface configuration and invariably demonstrates a prominent granular cell layer and marked hyperkeratosis. These different histologic patterns exist alone or intermingle with one another (5).
Esophageal condylomas demonstrate the cytologic changes characteristic of HPV infection, including giant cells, multinucleated cells, superficial koilocytosis, and anisonucleosis (Fig. 3.3). Disturbances in squamous cell maturation and keratinization are present, including hyperkeratosis, acanthosis, papillomatosis, and dyskeratosis. Papillomas are benign lesions with little or no malignant potential.
Papillomatosis
Papillomatosis consists of multiple minute esophageal squamous papillomas. It involves any part of the esophagus, most frequently affecting the distal esophagus. The lesion is rare,
appearing in <1% of endoscopic examinations. The papillomas appear as multiple, small, irregular, wartlike mucosal projections. Their histology resembles that seen in isolated papillomas. Esophageal papillomatosis results from chronic inflammation from HPV infection, gastroesophageal reflux, prolonged nasogastric intubation, or the use of esophageal self-expanding metal stents. One unusual patient had multiple polyps showing synchronous HPV-16 and -33 infections. She also had a gastric carcinoma and once the gastric carcinoma was resected, the esophageal polyps completely regressed (12).
appearing in <1% of endoscopic examinations. The papillomas appear as multiple, small, irregular, wartlike mucosal projections. Their histology resembles that seen in isolated papillomas. Esophageal papillomatosis results from chronic inflammation from HPV infection, gastroesophageal reflux, prolonged nasogastric intubation, or the use of esophageal self-expanding metal stents. One unusual patient had multiple polyps showing synchronous HPV-16 and -33 infections. She also had a gastric carcinoma and once the gastric carcinoma was resected, the esophageal polyps completely regressed (12).
TABLE 3.1 World Health Organization Classification of Esophageal Epithelial Tumors | |
|---|---|
|
TABLE 3.2 TNM Classification of Esophageal Carcinomas | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
Squamous Cell Carcinoma and its Precursors
Globally, esophageal SCC is the sixth most common cancer in males and the ninth most common in females (2). Esophageal SCCs show marked geographic (13) and ethnic
(2,13) differences. Esophageal SCC is relatively uncommon in the United States (3) and Western Europe. Its annual incidence is 2.2 per 100,000 among white men in the United States compared to 13.2 among black men (3); 18.2 per 100,000 in Normandy, France (13); and 8.2 per 100,000 in Shanghai, China and 183.8 per 100,000 in Linxian, China (13). The most prominent cluster of elevated cancer rates occurs in north central China on the border of Henan, Hebei, and Shanxi provinces. Northeast China is the eastern pole of the Asian belt of SCC that begins in eastern Turkey and extends through the southern states of the former Soviet Union, Iran, and Iraq (14). Other high-risk areas include Chile, the Transkei region of South Africa, Japan, and Brazil (13). Because many risk factors interact with one another, their individual effects are difficult to weigh; however, antecedent esophagitis is common to all patients with esophageal carcinoma. Migrants from high-risk to low-risk countries retain their high risk through the first generation, but fall to the level of the host country in the second generation (15). This decline in rates may be attributed to diminished exposure to the environmental carcinogens peculiar to the country of origin. The persistence of risk in first-generation migrants, however, suggests that the anatomic changes induced by these carcinogens are not reversed in the new environment.
(2,13) differences. Esophageal SCC is relatively uncommon in the United States (3) and Western Europe. Its annual incidence is 2.2 per 100,000 among white men in the United States compared to 13.2 among black men (3); 18.2 per 100,000 in Normandy, France (13); and 8.2 per 100,000 in Shanghai, China and 183.8 per 100,000 in Linxian, China (13). The most prominent cluster of elevated cancer rates occurs in north central China on the border of Henan, Hebei, and Shanxi provinces. Northeast China is the eastern pole of the Asian belt of SCC that begins in eastern Turkey and extends through the southern states of the former Soviet Union, Iran, and Iraq (14). Other high-risk areas include Chile, the Transkei region of South Africa, Japan, and Brazil (13). Because many risk factors interact with one another, their individual effects are difficult to weigh; however, antecedent esophagitis is common to all patients with esophageal carcinoma. Migrants from high-risk to low-risk countries retain their high risk through the first generation, but fall to the level of the host country in the second generation (15). This decline in rates may be attributed to diminished exposure to the environmental carcinogens peculiar to the country of origin. The persistence of risk in first-generation migrants, however, suggests that the anatomic changes induced by these carcinogens are not reversed in the new environment.
The male:female ratio varies from 1.5:1 in Linxian, China, to 17:1 in Normandy, France (13). In the United States, the age-adjusted incidence of esophageal cancer is highest among blacks (15 per 100,000) and Hawaiians (5,8) and is lowest among Filipinos (2.9) (16). The incidence of esophageal cancer increases with age and peaks in the 6th decade in high-risk areas (13). Patients 35 years or younger constitute approximately 7.4% of esophageal cancers in high-risk populations such as China. Time-trend studies show that incidence rates for SCC are stable or decreasing in most populations. For example, there has been a decrease in SCC incidence among U.S. blacks of 5.8% per year between 1992 and 2001 (17), while the rates of SCC in U.S. whites have fallen below that of esophageal adenocarcinoma (3). A decrease in smoking in Western countries (18), combined with an increased consumption of fresh fruits and vegetables, may account for this decline (19).
The incidence of esophageal SCC closely correlates with a low socioeconomic status whatever the level of risk in any population (20); childhood economic status is a predictor of SCC risk in the adult (14). This suggests that increased tobacco consumption (21) and poor nutrition associated with poverty are common denominators in the genesis of this cancer. The increased incidence in U.S. black males has been attributed to increased tobacco and alcohol use, but the deleterious effects of these agents are amplified by the absence of the protective effects of fruits and vegetables (19).
Etiology
Both genetic and environmental factors play a role in the genesis of esophageal SCC. Environmental factors associated with esophageal SCC and adenocarcinomas are summarized in Table 3.3.
Alcohol and Tobacco
Heavy consumption of strong liquors, such as whiskey and calvados, shows a dose-dependent increased SCC risk (19,22). The combined use of alcohol and tobacco incurs a multiplicative increase in the risk of SCC. In Brittany, the relative risk is 49.6 for nonsmokers with the highest alcohol intake, 7.8 for nondrinkers with the highest tobacco use, and 155.6 for men at the maximum consumption level of both substances (23). Persons at high risk for SCC due to alcohol and tobacco consumption and poor nutrition may be vulnerable to field cancerization of
the entire upper aerodigestive tract (UADT), including the esophagus (24). The frequency of developing a second UADT SCC in the 10 years following treatment of an index cancer is estimated to be from 5% to 40% (25). A prospective family study found the 10-year standardized incidence rates for esophageal cancer to be elevated after the diagnosis of an index cancer in the UADT (8.24) and lung (2.0) (26). Half of the multicentric cancers are synchronous. Most metachronous cancers develop within 3 years.
the entire upper aerodigestive tract (UADT), including the esophagus (24). The frequency of developing a second UADT SCC in the 10 years following treatment of an index cancer is estimated to be from 5% to 40% (25). A prospective family study found the 10-year standardized incidence rates for esophageal cancer to be elevated after the diagnosis of an index cancer in the UADT (8.24) and lung (2.0) (26). Half of the multicentric cancers are synchronous. Most metachronous cancers develop within 3 years.
TABLE 3.3 Environmental Risk Factors for Esophageal Carcinoma | ||||||||||||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| ||||||||||||||||||||||||||||||||||||||||||
Dietary/Personal Factors
Patients with esophageal SCC can be divided into two overlapping risk groups: Those who smoke and consume large amounts of alcohol and those whose diets lack green, leafy vegetables, citrus fruits, micronutrients, and trace elements (27). The missing trace elements include molybdenum, manganese, zinc, iron, silicon, barium, titanium, and magnesium. Mineral deficiencies in the soil lead to increased fungal invasion and mycotoxin food contamination. Calcium, riboflavin, vitamin A, and vitamin C deficiencies may also play a role in esophageal carcinogenesis, since these vitamins play a role in maintaining mucosal integrity and normal epithelial differentiation. Deficiencies in these substances render the esophageal mucosa vulnerable to carcinogens in foods, such as mycotoxins in the Transkei (28) and N-nitroso compounds in China (29).
The influence of dietary supplements on SCC cancer risk has been studied among high-risk Chinese communities. These micronutrients have included β-carotene, vitamin E, selenium, zinc, molybdenum, retinol, riboflavin, and vitamin C. Overall, the evidence shows that these supplements cause a small, or borderline, reduction in the SCC risk (27). Since exposure to dietary carcinogens begins in childhood, it is not surprising that micronutrient supplements show only modest protection from this cancer in the adults in high-risk populations.
Thermal injury from hot drinks including hot tea in East Asia and maté consumption in South America has long been identified as a risk factor for SCC (27,30). It is difficult to separate the effects of thermal injury from the constituents of tea and maté, but the many studies strongly suggest that hot drinks do impose a risk of developing SCC (27).
The nitrosamine content of foods varies widely in high- and low-risk areas of China. Fermented, moldy, pickled food that contains high levels of nitroso compounds is a dietary staple in high-risk areas (27). The low molybdenum content of the soil in these high-risk areas yields crops with a high nitrate content that may be converted to potentially carcinogenic nitroso compounds. Nitrosamines are potent alkylating agents that can produce various alkyl DNA adducts, particularly involving O6-methylguanine, which can preferentially mispair with thymine rather than cytosine, causing GC to AT mutations (31). O6-methylguanine-DNA methyl transferase (MGMT) is a primary defense against alkylase-induced carcinogenesis. Some SCC patients have MGMT inactivation via aberrant promoter methylation (32). Vitamin C inhibits this conversion.
The dietary factors that increase or decrease the SCC risk vary from culture to culture. Thus, heavy chili consumption is an independent risk factor for SCC in India (33). Capsaicin, the active component of chili and its metabolites, may be a proximate carcinogen/mutagen. In contrast, a Mediterranean diet would appear to lower the risk of SCC, even when associated with moderate to high alcohol consumption (34).
Environmental and Genetic Interactions
Genetic polymorphisms in enzymes that metabolize alcohol render some individuals vulnerable to its deleterious effects. Acetaldehyde, the first metabolite of alcohol, is eliminated by aldehyde dehydrogenase-2 (ALDH2) (35). ALDH2 polymorphisms influence blood acetaldehyde concentrations after drinking (36). An inactive mutant allele, ALDH2*2, is common in Orientals and is associated with increased circulating acetaldehyde levels, inducing painful facial flushing among occasional alcohol consumers (37). Alcoholics with this mutant allele develop tolerance to this reaction and the presence of this less active genotype in alcoholics enhances their SCC risk (38). The influence of alcohol on SCC risk may also be modified by an XRCCI polymorphism at codon 399. The XRCCI protein facilitates base excision repair or single-strand break repair. The odds ratio (OR) for SCC among alcoholics with the Arg/Arg XRCCI genotype compared to the Arg/Gln or Gln/Gln genotypes is 2.78 (1.15 to 6.67) (39). Recent data suggest that individuals with low selenium intake and ALDH2 Lys/Lys and XRCC1 399 Gln/Gln genotypes associate with an increased risk of developing SCC, especially in the presence of exposure to tobacco and alcohol (40).
Aromatic hydrocarbons in tobacco smoke require metabolic activation by phase I enzymes (CYP450s), and are then subject to detoxification by phase II enzymes (GSTM1) (41). Patients with the Val/Val CYP1A1 genotype are at increased risk of SCC (OR = 6.63, 95% confidence interval [CI], 1.86 to 23.7); this risk is enhanced when associated with GSTM1– (OR = 12.7, CI 95%, 1.97 to 81.8) (47). The cyclin D1 G870A polymorphism also influences the SCC risk in smokers (42). A study of North Chinese patients found that smokers with the G/G cyclin D1 phenotype have a lower risk of SCC than those with the G/A or A/A phenotypes.
Low folate and impaired folate metabolism have also been implicated in the development of gastrointestinal cancers. Genetic polymorphisms in 5,10-methylenetetrahydrofolate reductase (MTHFR) plays a central role in folate metabolism. This gene is polymorphic and the MTHFR 677TT genotype, which is associated with reduced enzymatic activity, significantly associates with increased esophageal SCC risk (43).
Occupational Factors
Certain occupations associate with an increased risk for SCC. These include warehouse workers and workers in miscellaneous food industries as well as those with occupational
exposure to asbestos, metal dusts, vulcanization products, asphalt, petrochemicals, and combustion products (44). Persons engaged in the production and distribution of alcoholic beverages also have an increased SCC risk as seen in the Calvados region of France (45).
exposure to asbestos, metal dusts, vulcanization products, asphalt, petrochemicals, and combustion products (44). Persons engaged in the production and distribution of alcoholic beverages also have an increased SCC risk as seen in the Calvados region of France (45).
Radiation Exposure
Radiation therapy increases esophageal SCC in patients treated for various malignancies. As an example, the risk following treatment for breast cancer increases starting 5 years after exposure (46). Most radiation-induced cancers arise in the upper and midesophagus and the tumors may be multifocal in nature.
Infections
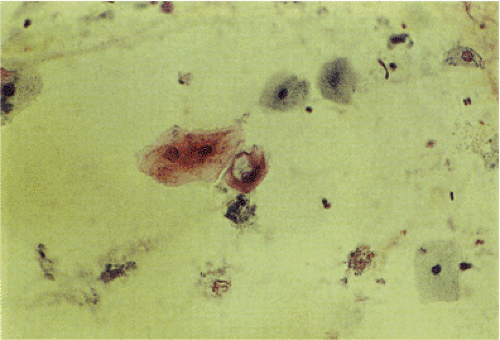
HPV DNA may be found in invasive SCC, areas of carcinoma in situ, the hyperplastic epithelium surrounding cancers, and histologically normal cells near these cancers (47). The areas with the highest incidence of HPV infections include China, Japan, and parts of South Africa. Although HPV types 6, 7, 9, 11, 13, 16, 18, 24, 30, 33, 51, 52, 57, and 73 have all been identified, HPV 16 is the most common (47). A p53 codon 72 polymorphism significantly (p = 0.001) associates with HPV-related esophageal cancers in northern China (48). Fifty-three percent of patients with HPV positive were Arg/Arg carriers, compared to 26% of HPV-negative cancers and 23% of controls. The presence of HPV infection may be suspected in cytology preparations, as shown in Figure 3.3.
Fungi frequently contaminate the grains and foodstuffs consumed in high-risk geographic areas. The fungi belong to many genera, but Fusarium and Aspergillus are the two most common and their respective mycotoxins, fumonisin B1 and aflatoxin, act as carcinogens. They are found on maize (corn), millet, and other cultivated grains in Linxian and on pickled vegetables. The fungi reduce nitrates to nitrites and promote the formation of nitrosamines (22).
Chronic Chagas disease, resulting from infection by Trypanosoma cruzi, causes achalasia and associates with SCC development (49). Since the infection is frequently acquired in early childhood, the resulting carcinoma may appear as early as the 3rd decade of life.
Inherited Risk Factors
The autosomal dominant familial syndrome palmoplantar keratoderma (PPK) (tylosis) predisposes patients to the development of esophageal SCC. PPK results in defective mucosal keratinization (50) and altered mucosal integrity, increasing susceptibility to environmental mutagens. The mean age of onset for esophageal malignancies in these patients is 61 years. Members of PPK families have a 50% risk of developing esophageal cancer by age 50 and a 90% to 95% risk of developing esophageal cancer by age 65 (50). These patients also have an increased risk of melanoma, breast and lung cancer, leukemia, hepatomas, malignant lymphomas, and colorectal adenomas (50,51). The presence of multiple carcinomas involving the oral cavity, tongue, oropharynx, and stomach suggests that exposure to a common environmental agent contributes to the genesis of all of the tumors. PPK patients who smoke are particularly likely to develop SCC (51). PPK results from a mutation in the tylosis in esophageal cancer (TOC) region of chromosome 17q25 (52). Loss of heterozygosity (LOH) of polymorphic microsatellite markers located near the TOC locus also occurs in sporadic esophageal cancers (52,53). PPK patients develop chronic esophagitis, followed by dysplasia, carcinoma in situ, and invasive carcinoma. Other histologic changes include abnormal keratinocyte maturation, the presence of basophilic inclusions, and surface keratinization (54). The neoplastic squamous cell lesions that develop resemble those arising in the absence of tylosis.
The recessive form of dystrophic epidermolysis bullosa due to a type VII collagen mutation is a less common source of inherited esophageal SCC (55). This condition is most common among people of Northern European origin.
Predisposing Diseases
Chronic esophagitis is the most common SCC precursor (56). The esophagitis has many causes, as discussed in Chapter 2. SCC arising in association with GERD originates in the mucosa bordering the upper limits of BE (57). The SCC associated with caustic burns emerges 30 to 45 years after the initial injury, usually in the midesophagus (58). Motility disorders that increase the risk of SCC include achalasia and scleroderma. Patients with achalasia develop esophagitis due to postprandial retention of solid foods (59). Food retention also increases the risk of SCC in diverticula, including Zenker (60) and the epiphrenic diverticula. The achlorhydria that accompanies autoimmune atrophic gastritis associates with an elevated SCC risk (61), as does the multifocal gastritis caused by CagA-positive Helicobacter pylori. This effect is strongest among patients with gastric atrophy, as measured by low pepsinogen group I levels (62). The association is supported by the parallel decline in the incidence of esophageal SCC and H. pylori–induced stomach cancer in migrants from Japan to the United States (63). The esophagitis that accompanies Plummer-Vinson syndrome also associates with an increased risk of SCC in the hypopharynx and cervical esophagus (64). There is also an association with celiac disease (64).
Dysplasia
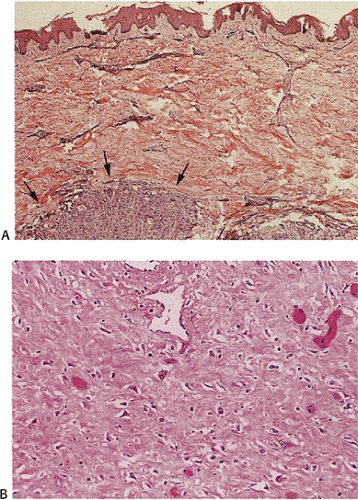
Esophageal SCC passes through the sequence of chronic esophagitis, low-grade and high-grade dysplasia (also know as intraepithelial neoplasia), and invasive carcinoma. As a result, histologic or cytologic specimens may contain a constellation of histologic abnormalities, including normal, or
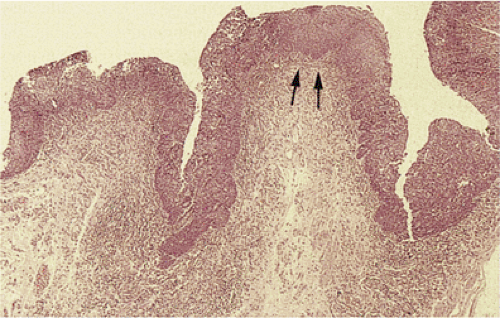
near-normal esophageal mucosa, atrophy; esophagitis; parakeratosis; dyskeratosis; basal cell hyperplasia; simple hyperplasia; mixed basal spinous hyperplasia; variable degrees of dysplasia; and invasive cancer. In general, the epithelium increases in thickness and the papillae elongate as the process evolves (Figs. 3.4 and 3.5). Dysplasia, defined as the presence of unequivocally neoplastic squamous cells that are confined to the mucosa above the basement membrane, is the immediate precursor lesion for SCC. Dysplasia was traditionally classified into mild, moderate, and severe degrees. However, since interobserver agreement in distinguishing the three grades is generally poor, most favor a two-tier
system of low-grade and high-grade dysplasia. The latter classification scheme includes epithelial changes previously classified as carcinoma in situ. The higher the grade of dysplasia, the greater is the likelihood that the lesion will evolve into an invasive cancer.
near-normal esophageal mucosa, atrophy; esophagitis; parakeratosis; dyskeratosis; basal cell hyperplasia; simple hyperplasia; mixed basal spinous hyperplasia; variable degrees of dysplasia; and invasive cancer. In general, the epithelium increases in thickness and the papillae elongate as the process evolves (Figs. 3.4 and 3.5). Dysplasia, defined as the presence of unequivocally neoplastic squamous cells that are confined to the mucosa above the basement membrane, is the immediate precursor lesion for SCC. Dysplasia was traditionally classified into mild, moderate, and severe degrees. However, since interobserver agreement in distinguishing the three grades is generally poor, most favor a two-tier
system of low-grade and high-grade dysplasia. The latter classification scheme includes epithelial changes previously classified as carcinoma in situ. The higher the grade of dysplasia, the greater is the likelihood that the lesion will evolve into an invasive cancer.
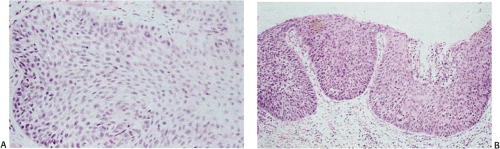 FIG. 3.5. Dysplasia of the esophageal epithelium. A: Moderate dysplasia. B: Severe dysplasia–carcinoma in situ. |
The following facts support the concept that dysplasia is a step in SCC development. The frequency of dysplasia correlates with the level of risk. For example, endoscopically screened subjects in high-risk Linxian, China, show high-grade dysplasia in 7.9% of men and 9.8% of women. This contrasts with 2.4% and 2.5% in intermediate-risk Argentinean men and women, respectively, and 0% of screened subjects in low-risk areas of China (14). The age distribution of dysplasia and cancer supports a continuous progression from mild dysplasia to severe dysplasia to invasive carcinomas. Follow-up studies show that invasive cancers develop in areas of dysplasia. When assessed prospectively, 20% of 500 Chinese people with high-grade dysplasia progressed to carcinoma, compared with only 0.12% of 11,011 individuals with a normal mucosa (14). Areas of dysplasia share similar molecular abnormalities with the invasive cancers adjacent to them.
As in other tumors, there is a sequential acquisition of molecular abnormalities as the lesions progress from early precancerous lesions to invasive carcinoma (1). Those that are thought to be important are shown in Figure 3.6. A biopsy study from Chinese patients with dysplasia found genetic instability, including LOH and microsatellite instability (MSI) in the precursor lesions (65). LOH was identified at ten different markers on chromosomes 3p, 5q, 9p, 9q, 13q, 15q, and 17p. This study also found MSI in both low-grade and high-grade dysplasia, with its frequency increasing with the severity of the process. p53 mutations can be found in dysplasia and in situ cancer (66). The same mutations are present in the adjacent invasive cancer, indicating that p53 mutations are an early event in esophageal carcinogenesis. A Japanese study of field carcinogenesis found different p53 mutations in synchronous multicentric SCCs, but the identical p53 mutations were found in the dysplastic epithelium adjacent to each invasive cancer (67,68). p53 mutations may also be found in areas of transitions from esophagitis to dysplasia and are among the earliest changes seen in the development of esophageal SCC (1).
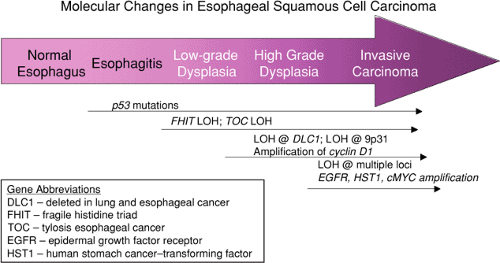 FIG. 3.6. Diagram of the progressive acquisition of molecular abnormalities as the epithelium progresses from normal to invasive cancer. |
Areas of dysplasia generally remain symptomless, and it takes 3 to 5 years for carcinoma in situ to progress to more advanced stages of the disease. Up to 25% of patients with squamous dysplasia will develop cancer within 8 years. Because of the stepwise progression of SCC, cytologic esophageal screening has proven to be cost effective in asymptomatic patients in the high-risk areas. Cytology examination of 12,877 subjects in a high-risk Chinese population found high-grade dysplasia in 6% of cases and carcinoma in 3% (69). Population-based screening programs for esophageal cancer are not applicable to low-risk areas, such as North America or Europe. Selective cytologic screening of high-risk individuals, however, such as participants in alcohol abuse programs, may discover SCC at an earlier stage of progression than is usually the case in Western countries, as shown by Japanese studies that targeted alcoholics using endoscopy and iodine staining (70).
Dysplasia has several endoscopic appearances including a friable, irregular erosion and a raised polypoid lesion, with or without erosions or a hyperemic roughening that bleeds easily. Dysplasia usually involves the middle and lower esophagus and does not affect motility. Grossly visible areas of dysplasia vary in size from 0.5 to 5 cm in diameter. The dysplasia may be scanty in type with well-defined margins, extensive, or multifocal. Extensive lesions have ill-defined
margins. The gross changes may be very subtle, showing only a slight mucosal depression, but mucosal staining with Lugol facilitates the detection of dysplasia since the normal mucosa is stained but the dysplastic mucosa is not (1). Dysplasia may merge with or be separate from an invasive cancer. Dysplasia is more likely to be identified in small or early cancers than in large or advanced neoplasms, since large cancers are more likely to overgrow their precursors.
margins. The gross changes may be very subtle, showing only a slight mucosal depression, but mucosal staining with Lugol facilitates the detection of dysplasia since the normal mucosa is stained but the dysplastic mucosa is not (1). Dysplasia may merge with or be separate from an invasive cancer. Dysplasia is more likely to be identified in small or early cancers than in large or advanced neoplasms, since large cancers are more likely to overgrow their precursors.
Histologic examination is necessary to diagnose dysplasia. Dysplasia is characterized by both architectural and cytologic abnormalities. A disorderly proliferation of immature cells with hyperchromatic nuclei, abnormally clumped chromatin, and pleomorphic nuclei is present (Fig. 3.4). The cells typically have an increased nuclear:cytoplasmic ratio and demonstrate loss of polarity. The nuclei are frequently overlapping. Mitoses are frequent and are often abnormal. The dysplasia is considered to be low grade when the abnormal cells are limited to the middle third of the mucosa and high grade when they extend to the upper third of the epithelium or involve the full mucosal thickness and show more epithelial atypia than low-grade lesions. In carcinoma in situ (the high end of the spectrum of high-grade dysplasia), atypical cells extend through the full thickness of the epithelium without evidence of surface maturation. Dysplastic cells may also extend into the underlying submucosal glands and ducts. While ductal extension predisposes to deeper penetration (71), this finding by itself does not constitute invasion. Dysplastic squamous cells may also spread in a pagetoid manner into the adjacent normal esophageal mucosa. The pagetoid cells stain positively with low-molecular-weight keratin, do not stain for mucus, and have a high proliferative index.
Acanthotic epithelial buds containing atypical epithelial cells occur in both low-grade and high-grade dysplasia. They appear regular and the same size or vary in size, shape, length, and width (Fig. 3.5). Irregular buds are most common in areas of severe dysplasia adjacent to invasive cancer (72) and microinvasive cancer tends to develop from their tips. Dysplasia associated with HPV infection shows koilocytotic changes. Lymphocytic infiltrates in the surrounding lamina propria are common and correlate with the severity of the dysplasia.
Japanese and Western pathologists differ in their diagnostic criteria for esophageal squamous cell lesions. Invasion is the most important diagnostic criterion for a diagnosis of carcinoma for Western pathologists, whereas nuclear and structural features are more important for Japanese pathologists. In Japan, esophageal squamous cell carcinomas are diagnosed based on nuclear grade, and include cases judged to be noninvasive, low-grade dysplasia in the West. This difference may contribute to widely variant incidence rates and predictions of prognosis. In an effort to address these differences, the Vienna Classification was developed (Table 3.4) (73), although it has not been widely adopted. It corresponds to the treatment recommendation of local tumor ablation.
There are several diagnostic pitfalls with respect to early squamous neoplasias. Dysplasia must be distinguished from regenerative changes or areas of pseudoepitheliomatous hyperplasia. Herpes esophagitis, chemotherapy, or radiotherapy and areas of regeneration adjacent to ulcers may induce histologic changes that may be mistaken for early squamous neoplasia. The differences between regenerative and neoplastic changes are summarized in Table 3.5. Inflammation or ulceration, if extensive, may help identify the epithelium as regenerative in nature. However, caution must be exercised because dysplastic epithelium may become ulcerated or inflamed. If one is unable to distinguish between a truly dysplastic lesion and one that is regenerative in nature, one can use the term indefinite for dysplasia to diagnose the changes that are present. Subsequent biopsies, particularly those obtained once the inflammation has been treated, often help clarify the nature of the underlying process. Immunostaining for p53 may also help distinguish
dysplasia from reactive changes. While this test is by no means specific, the presence of large numbers of p53 immunoreactive cells (as opposed to only isolated positive cells) is much more likely to be present in areas of dysplasia than in reactive lesions.
dysplasia from reactive changes. While this test is by no means specific, the presence of large numbers of p53 immunoreactive cells (as opposed to only isolated positive cells) is much more likely to be present in areas of dysplasia than in reactive lesions.
TABLE 3.4 Vienna Classification | |
|---|---|
|
TABLE 3.5 Features Useful in Distinguishing between Reactive and Neoplastic Squamous Epithelium | |||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| |||||||||||||||||||||||
Both radiation and/or chemotherapy, particularly in the setting of neoadjuvant therapy, may induce mucosal changes that mimic dysplasia. Individual cells may have irregular contours and contain hyperchromatic nuclei as well as abnormal mitotic figures. Often the latter have a ring shape, a feature not found in neoplastic cells, which helps avoid overdiagnosing dysplasia or carcinoma in this setting.
A final pitfall in the diagnosis is the examination of biopsies that are not representative of the mucosal changes. This generally results from superficial biopsies that do not contain lamina propria so that areas of invasive carcinoma cannot be excluded and very superficial biopsies that may miss pagetoid spread of a tumor along the basal part of the mucosa.
Early Esophageal Cancers
The term early esophageal cancer is used by the Japanese and Chinese to designate a neoplastic process that is confined to the mucosa or submucosa, regardless of the nodal status. For these pathologists, early esophageal cancers include both areas of dysplasia and superficially invasive cancer with and without nodal metastases. The small proportion of early cancers that become symptomatic and/or have abnormal radiographic features are most likely to be protruding lesions. The prognosis in early lesions is good and progression to advanced cancer is slow (74). It takes a little more than 1 year to progress from mucosal involvement to submucosal invasion, 6.6 ± 3.2 months to progress from submucosal invasion to advanced disease, and 21.1 ± 6.8 months to progress from mucosal to advanced disease (75).
Early cancers may be multicentric or consist of large fields of invasive or microinvasive cancer associated with varying degrees of epithelial dysplasia. Several macroscopic types of early carcinoma are recognized: Flat, coarse, verrucous, polypoid, and ulcerating infiltrating (76). Most appear as areas of redness, plaques, or maplike erosions. The frequency of multicentric esophageal SCC ranges from 7% to 28% (76,77). Flat, early lesions may be difficult to detect endoscopically or in resected specimens. Lugol staining of the specimen highlights the abnormal areas and allows one to assess the extent of the disease and to detect the presence of multiple lesions.
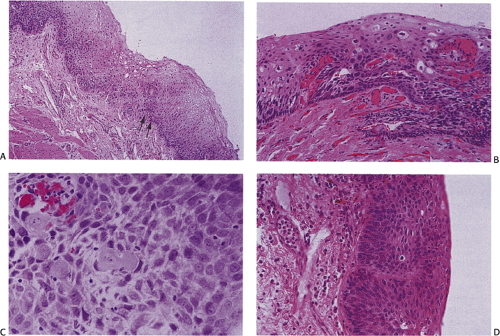
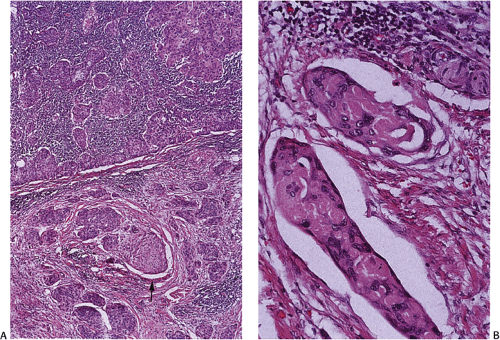
Several histologic patterns of early esophageal cancer exist: (a) conventional SCC, (b) SCC with basaloid features and expansile growth, and (c) SCC with spindle cell features (76,78). Tumor cells invading the submucosa appear larger than the dysplastic cells. Areas of dysplasia often surround the invasive foci. Basaloid tumors tend to show prominent peripheral nuclear palisading and the invasive cells tend to appear smaller than those of ordinary SCC. Basaloid tumors tend to be superficial and lack metastases, thus having a better prognosis than other histologic types (78). The earliest invasive lesions appear to drop off the base of the mucosa. The overlying mucosa may or may not be involved by full-thickness replacement by intraepithelial neoplasia (Fig. 3.7). Progression from noninvasive to invasive disease associates with an increased lymphocytic reaction (Fig. 3.7).
Identifying superficially invasive cancers in resection specimens is usually not a diagnostic problem. However, the interpretation of small endoscopic biopsies may be challenging. The distinction of malignancy from pseudoepitheliomatous hyperplasia in regenerating squamous epithelium in the setting of esophagitis poses the greatest difficulty. Regenerating squamous cells generally do not show abnormal mitoses or loss of polarity.
When a cancer is superficial and not associated with nodal metastases, endoscopic mucosal resection (EMR) may be the treatment of choice since it incurs less morbidity and a smaller risk of mortality than esophagectomy. This procedure is now widely used in Japan. Single or multiple areas of the mucosa unstained by iodine identify resection targets. Recurrence after EMR varies from 2% to 4%, but may be treated by repeat EMR.
Patient survival with SCC correlates with the extent of the tumor, and there are several systems used to assess the stage of this disease. The different systems shown in Table 3.6 have been used to stage the SCCs and each has advantages in certain situations. The T (tumor) N (node) M (metastasis) system is used by the American Joint Committee for Cancer Staging and End Results (AJCC) (79) and the WHO classification (1). When preceded by a lowercase “p”, the TNM values are based on pathology findings. When preceded by lowercase “yp”, the TNM values represent pathology review of a tumor that has been previously treated (80). A simplified system used by the Surveillance, Epidemiology, and End Results (SEER) registries is frequently used in epidemiologic studies of incidence and
mortality since it generates data comparable to those from the large, population-based international registries that are published by the International Agency for Cancer Research (13). Tachibana et al. (81) proposed a modified Dukes system to bypass international variations in the use of the TNM system. One variable not included in these systems is tumor size even though this is an independent predictor of 1- and 2-year survivals among patients with node-negative cancers (82). The number of involved lymph nodes also correlates with mortality (82).
mortality since it generates data comparable to those from the large, population-based international registries that are published by the International Agency for Cancer Research (13). Tachibana et al. (81) proposed a modified Dukes system to bypass international variations in the use of the TNM system. One variable not included in these systems is tumor size even though this is an independent predictor of 1- and 2-year survivals among patients with node-negative cancers (82). The number of involved lymph nodes also correlates with mortality (82).
TABLE 3.6 Comparison of Staging Systems for Esophageal Squamous Cell Cancer | |||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| |||||||||||||||||||||||||||||||||
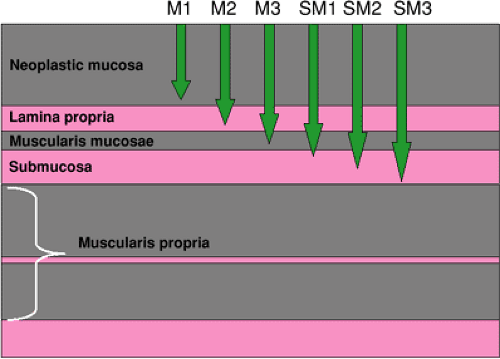
In countries such as China and Japan, where a large proportion of SCCs are diagnosed at an early stage, a more discriminating assessment of prognosis of early cancers is achieved by dividing them into three levels of mucosal and submucosal penetration (Fig. 3.8) (83). The deeper the penetration, the greater the probability is of lymph node metastases, as shown by Araki et al. (84), who found nodal spread in 35.7% of sm3 cancers, compared with 8.3% of sm1 lesions. This staging system is commonly used to evaluate EMR specimens. Other Japanese studies have also found lymph node metastases in approximately 30% of tumors that penetrate to the submucosa (84).
Multivariate analysis indicates that factors that increase the relative risk of recurrence with submucosal cancer are intramural metastases, vascular invasion, and nodal metastases (85). The postresection 5-year survival rates of patients with submucosal SCC vary from 44% to 96%, with the most favorable prognosis occurring in patients without vascular invasion, intramural metastases, or nodal involvement. Patients with all three of these have the least favorable prognosis (85). If metastases are found in a patient with esophageal high-grade dysplasia, or if one detects vascular and/or lymphatic vessel invasion, an undetected invasive cancer is likely to be present. Resection specimens should be extensively sampled to find the invasive focus. If none is found the possibility remains that an invasive focus remains in the patient.
Superficial Spreading Carcinoma
Superficial spreading carcinoma is defined as a tumor >5 cm in length consisting mainly of intraepithelial carcinoma with or without limited submucosal invasion. The tumor can measure up to 14 cm in length and is frequently located in the midesophagus. Like other early squamous cell lesions, the lesions may be superficial and flat, slightly elevated, slightly depressed, or superficial and distinctly depressed (86). These tumors may show well-differentiated or moderately differentiated squamous cell histologies or may be basaloid in appearance. Superficial spreading carcinomas are likely to be multicentric. These lesions are important to recognize due to the difficulty in establishing clear resection margins at the time of esophagectomy (87). The mean age of patients with superficial spreading esophageal squamous carcinoma is 56 to 63 years, with the lesion being most common in females.
Advanced Squamous Cell Carcinoma
Clinical Features
Esophageal SCC usually grows slowly. The patients may remain asymptomatic with invasive disease because the esophagus is highly distensible and tumors can grow to a considerable size before the lumen becomes sufficiently narrowed to produce symptoms. Symptomatic esophageal SCC presents with dysphagia, odynophagia, weight loss, coughing, choking, pain, and dehydration (88). Frequently patients do not complain of dysphagia, but make subtle changes in their eating habits without realizing it. Other symptoms include fever, anemia, hematemesis, melena, hoarseness, or the sensation of food “becoming stuck in the throat.” A persistent hiccup may indicate the presence of laryngeal nerve paralysis or aspiration, ominous signs of advanced disease. Local tumor extension beyond the esophagus often leads to
substernal or high back pain. Aortic erosion results in rapid exsanguination. Aspiration of esophageal contents through a tracheoesophageal fistula is a common cause of death in patients with advanced SCC. Palpable lymph nodes in the cervical or supraclavicular regions may be noted.
substernal or high back pain. Aortic erosion results in rapid exsanguination. Aspiration of esophageal contents through a tracheoesophageal fistula is a common cause of death in patients with advanced SCC. Palpable lymph nodes in the cervical or supraclavicular regions may be noted.
Patients may also develop various paraneoplastic syndromes. Tumor-induced hypercalcemia is common (89). It results from the release of calcium from the bones due to tumor production of parathyroid hormone–related protein (PTHrP) (90). The presence of hypercalcemia is a poor prognostic sign (91). A hypertrophic osteoarthropathy affects some patients with esophageal SCC (92), but this may result from concomitant chronic obstructive lung disease, which shares an association with heavy tobacco consumption. An acute vasculitis developed in a patient with stage II SCC, a phenomenon attributed to circulating immune complexes or tumor-associated antigens (93). The vasculitis went into remission after tumor resection.
The AJCC divides the esophagus into three compartments (79). The cervical esophagus extends from the pharyngoesophageal junction to the thoracic inlet, approximately 18 cm from the incisors. The middle esophagus extends from the thoracic inlet to a point 10 cm above the GEJ, approximately 31 cm from the incisors and at the level of the lower edge of the eighth thoracic vertebra. The lower esophagus extends from this point to the GEJ, or approximately 40 cm from the incisors. Carcinomas may develop in both the hypopharynx and cervical esophagus in men who are heavy consumers of alcohol and tobacco. SCCs complicating the Plummer-Vinson syndrome arise in the area of the cricopharyngeus muscle in women (male:femal ratio = 1:10) with a median age of 45 to 50 years. Cancers that appear after radiation therapy for breast cancer usually develop in the upper and midesophagus. Most SCCs in high-risk populations develop in the middle and lower thirds of the esophagus.
Pathologic Features
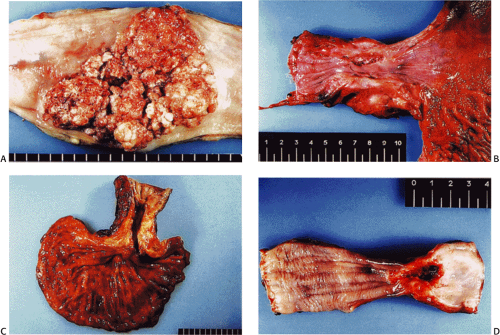
Esophageal SCCs appear as fungating, ulcerating, infiltrating, or stenotic lesions. Mixed gross growth patterns also occur. Advanced tumors may measure up to 10 cm in length. Fungating cancers present either as large, intraluminal, variably ulcerated masses with raised, everted margins or less commonly as polypoid, irregular, bulky tumor masses (Fig. 3.9).
The lesions may have sharply defined borders; intramural submucosal extension may be grossly inapparent. The extent of tumor infiltration at the tumor base varies and does not necessarily reflect the size of the protruding mass. Ulcerating cancers have irregular, noninverted, and sometimes serpiginous edges, and a shaggy, hemorrhagic central crater. The ulcers lie along the longitudinal esophageal axis and the tumors may extend into the surrounding viscera. Infiltrating carcinomas cause esophageal wall thickening. The esophagus appears rigid with slitlike areas of stenosis. The proximal esophagus dilates. Rarely, the pattern resembles the linitis plastica pattern seen in gastric carcinoma. Papillary or verrucous lesions usually develop in the proximal esophagus. Their differential diagnosis includes a papilloma, sarcomatoid carcinoma, and verrucous carcinoma.
The lesions may have sharply defined borders; intramural submucosal extension may be grossly inapparent. The extent of tumor infiltration at the tumor base varies and does not necessarily reflect the size of the protruding mass. Ulcerating cancers have irregular, noninverted, and sometimes serpiginous edges, and a shaggy, hemorrhagic central crater. The ulcers lie along the longitudinal esophageal axis and the tumors may extend into the surrounding viscera. Infiltrating carcinomas cause esophageal wall thickening. The esophagus appears rigid with slitlike areas of stenosis. The proximal esophagus dilates. Rarely, the pattern resembles the linitis plastica pattern seen in gastric carcinoma. Papillary or verrucous lesions usually develop in the proximal esophagus. Their differential diagnosis includes a papilloma, sarcomatoid carcinoma, and verrucous carcinoma.
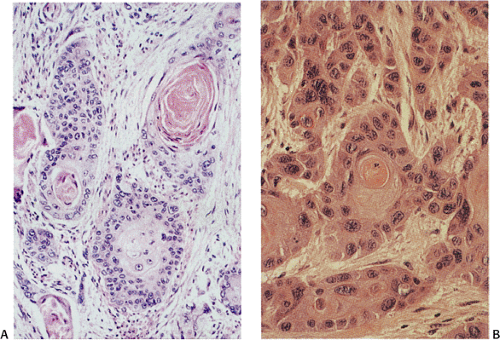 FIG. 3.10. Well-differentiated keratinizing squamous cell carcinoma. A: Note the infiltrating nests with prominent central keratinization. B: The high nuclear-to-cytoplasmic ratio is appreciated. |
Biopsies cannot determine the extent of invasion, but they yield a positive diagnosis in 81% to 100% of cases, depending on the number of biopsies obtained. Endoscopic ultrasonography (EUS) may identify tumor penetration to the submucosa or muscularis propria in up to 80% of early cases (94). EUS is most useful in identifying the superficial lesions that are best treated by surgery alone. Detection of nodal involvement is more accurate with EUS than with tomography (95). Fine needle aspirations enhance the ability of EUS to detect nodal disease, yielding an accuracy of more than 90% (96).
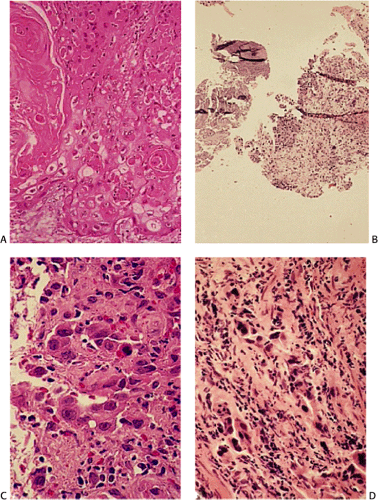
SCCs arise from areas of pre-existing dysplasia and invade through the basement membrane into the lamina propria or deeper tissues. Histologically, ordinary SCCs show varying grades of differentiation ranging from a well-differentiated keratinizing carcinoma containing well-formed squamous cell nests and keratin pearls to undifferentiated tumors without recognizable keratin or prickle cells. The tumors are classified into well-, moderately, and poorly differentiated lesions based on how closely they resemble mature nonneoplastic squamous cells (Figs. 3.10, 3.11, and 3.12). As the lesions become less mature, they show progressive degrees of pleomorphism and loss of keratinization and intercellular bridges. The degree of keratinization and the prominence of intercellular bridges inversely correlate with the degree of cytologic atypia and nuclear pleomorphism, although one occasionally sees marked keratinization of highly atypical cells. Most tumors are well- to moderately differentiated lesions. Well-differentiated keratinizing carcinomas have a high proportion of large differentiated squamous cells (Fig. 3.10) and a low proportion of small basal-type cells, which are typically located at the periphery of the tumor cell nests. The tumors contain squamous cell nests, dyskeratotic cells, and keratin pearls. Less well-differentiated tumors consist of cellular masses containing
polygonal, round, fusiform, or, rarely, small nonkeratinizing cells. Poorly differentiated carcinomas typically contain an abundance of basaloid cells and the degree of differentiation often varies throughout the tumor. Most poorly differentiated SCCs contain some foci of squamous differentiation such as dyskeratotic epithelial nests, keratin pearls, or intercellular bridges. They generally appear as invasive sheets of cells with prominent areas of central necrosis. Occasionally, areas of individual cell necrosis in poorly differentiated tumors produce a pseudoacinar pattern, but mucicarmine stains are negative. Typically the degree of differentiation varies throughout the tumor. In undifferentiated lesions, one may not recognize keratin or prickle cells, and one has difficulty identifying the tumor as squamous in nature. Most tumors contain at least focal histologically identifiable squamous differentiation with evidence of epithelial nests, pearls, or intercellular bridges. Cytokeratin 14 immunostains may help identify a tumor as being squamous cell in origin (97).
polygonal, round, fusiform, or, rarely, small nonkeratinizing cells. Poorly differentiated carcinomas typically contain an abundance of basaloid cells and the degree of differentiation often varies throughout the tumor. Most poorly differentiated SCCs contain some foci of squamous differentiation such as dyskeratotic epithelial nests, keratin pearls, or intercellular bridges. They generally appear as invasive sheets of cells with prominent areas of central necrosis. Occasionally, areas of individual cell necrosis in poorly differentiated tumors produce a pseudoacinar pattern, but mucicarmine stains are negative. Typically the degree of differentiation varies throughout the tumor. In undifferentiated lesions, one may not recognize keratin or prickle cells, and one has difficulty identifying the tumor as squamous in nature. Most tumors contain at least focal histologically identifiable squamous differentiation with evidence of epithelial nests, pearls, or intercellular bridges. Cytokeratin 14 immunostains may help identify a tumor as being squamous cell in origin (97).
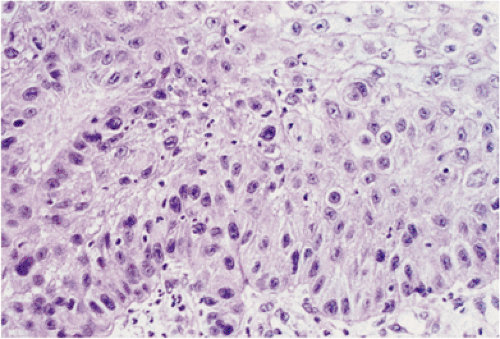 FIG. 3.11. Moderately differentiated invasive squamous cell carcinoma. Note the absence of marked atypia, keratinization, and pearl formation. |
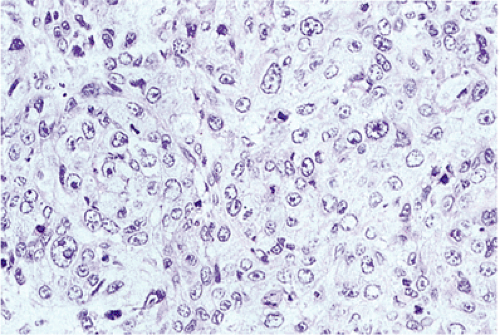 FIG. 3.12. Poorly differentiated invasive squamous cell carci-noma. Note the high degree of atypia and the brisk mitotic index. |
As the tumor cells infiltrate the esophageal wall, they may form sheetlike nests with rounded margins or they may have an asteroid shape with spiculated margins. Tumors with an asteroid configuration are more likely to show deep penetration, lymphatic permeation, nodal metastases, and desmoplasia and have a worse prognosis than those with rounded borders (98). Tumors with downward penetration are more likely to show vascular and lymphatic invasion (Fig. 3.13).
Patients treated preoperatively with chemotherapy or chemoradiation may show complete tumor ablation, partial regression with a reduced tumor:stroma ratio, or residual unaltered tumor cells, sometimes resulting in a stricture (Figs. 3.14 and 3.15). Tissues examined within 3 to 6 days of preoperative treatment show pronounced apoptotic changes, with or without necrosis. Most tumor cells appear extensively degenerated. The tumor may completely disappear, creating a
partially re-epithelialized ulcer containing granulation tissue heavily infiltrated by lymphocytes. Calcification may be present. Fibrosis in the muscularis propria causes shrinkage of the muscle bundles. Tumor regression can be graded (99), although this is not commonly done.
partially re-epithelialized ulcer containing granulation tissue heavily infiltrated by lymphocytes. Calcification may be present. Fibrosis in the muscularis propria causes shrinkage of the muscle bundles. Tumor regression can be graded (99), although this is not commonly done.
Cytologic Features
Cytologic evaluation of the esophagus is important as noted above. Exfoliated malignant cells from intraepithelial squamous cell carcinomas may be obtained by means of esophageal washings. These cells resemble those of carcinoma in situ of the cervix without evidence of keratinization. Abundant cell samples are usually obtained from SCCs. Paradoxically, large fungating growths frequently associate with inadequate material; false-positive results are rare in this setting. Well-differentiated carcinomas shed highly atypical and partly keratinized cells with bizarre shapes, anisocytosis, and hyperchromatic nuclei (Fig. 3.16). Malignant pearl formations may be observed. Moderately and poorly differentiated squamous cell carcinomas exfoliate single or clustered immature atypical cells with increased nuclear:cytoplasmic ratios and either pale nucleoli or dark angulated nuclei containing large amounts of heterochromatin (Fig. 3.17).
Tumor Spread
SCC spreads within the esophageal wall, invading the muscularis propria and extending into the periesophageal tissues. Mediastinitis, pleural fistulas, and empyema may develop. Depending on tumor location, invasion into the trachea, bronchi, aorta, pleural cavity, lung, thyroid, lymph nodes, pericardium, major vessels, and/or nerves occur. Malignant tracheoesophageal fistulas develop in up to 28% of patients (100). Eventually the tumors become fixed to surrounding structures, including the diaphragm, mainstem bronchi, aortic arch, or the great veins of the neck. At this point, the lesion is unresectable.
Intramural intralymphatic vascular spread is common; lymphatic extension produces submucosal nodules distant from the main tumor in up to 16% of esophageal carcinomas (85). The esophageal lymphatics drain into the mediastinal lymphatics leading to regional spread early in the disease. In Western populations, up to 61% of patients show local extension and nodal involvement at the time of diagnosis (101). Cervical nodes become involved in 19% to 60% of cases, mediastinal nodes in 21% to 64%, and abdominal nodes in 47%. Tumors of the cervical region metastasize to infraclavicular, peritracheal, supraclavicular, paraesophageal, and posterior mediastinal lymph nodes. Tumors of the upper and midesophagus commonly involve the paraesophageal, posterior, and tracheobronchial nodes. Lower esophageal tumors disseminate to paraesophageal, celiac, and splenic and hepatic artery lymph nodes. However, the distribution of nodal metastases does not always reflect the site of the primary tumor, since 40% of cervical esophageal tumors metastasize to abdominal nodes; 38% of lower esophageal carcinomas metastasize to cervical nodes (101). Extrathoracic nodal metastases represent distant metastases. The number of positive nodes is higher in distal cancers than in upper or midesophageal cancers. Simultaneous metastases to lymph nodes in the neck, mediastinum, and abdomen occur far more commonly in lower esophageal cancers than in upper and midesophageal cancers. In some cases, the appearance of a supraclavicular, cervical, or abdominal metastasis may precede detection of the primary tumor.
Hematogenous metastases occur late in the disease, usually following nodal metastases. The most common metastatic sites include liver (35% to 72%), lungs (20% to 60%), adrenals (35%), kidneys (25% to 26%), bones (9% to 20%), adrenal gland (5%), peritoneum (2%), and, rarely, the central nervous system (2%) (101).
Molecular Alterations
SCC induction and progression result from genetic and epigenetic changes in genes controlling cell cycling, cell growth, DNA repair, and tumor dissemination (see Fig. 3.18). A brief summary of immunostains that may be useful in the diagnosis of dysplasia and in determining the prognosis of esophageal SCC follows. Abnormalities in p53 expression are common in esophageal SCCs occurring early in tumor development (102). A significantly greater frequency of p53 expression occurs in high-grade dysplasia versus low-grade dysplasia and in low-grade versus normal esophageal mucosa (103). p53 expression had no influence on survival in one study (104), was a marker for radioresistance in another study (105), and in another had no predictive value in patients receiving either chemoradiation therapy or radiation alone (106). Cyclin D overproduction or production at the wrong time may stimulate
inappropriate cell division. Tumors showing cyclin amplification tend to invade deeply (107). Cyclin D overexpression increases in frequency from 22% in well-differentiated cancers to 54% in poorly differentiated tumors (107), but is not a useful prognostic marker. Cyclin B1 expression, however, associates with poor prognosis in both univariate and multivariate analyses (108).
inappropriate cell division. Tumors showing cyclin amplification tend to invade deeply (107). Cyclin D overexpression increases in frequency from 22% in well-differentiated cancers to 54% in poorly differentiated tumors (107), but is not a useful prognostic marker. Cyclin B1 expression, however, associates with poor prognosis in both univariate and multivariate analyses (108).
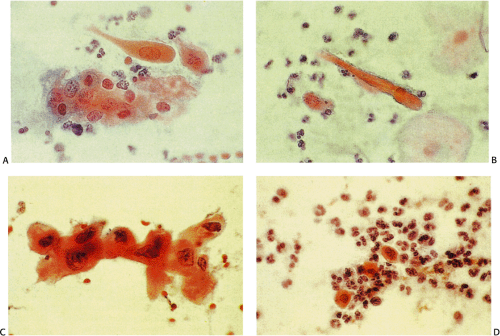 FIG. 3.16. Well-differentiated squamous cell carcinoma of the esophagus. A–D: Variably shaped cells are found singly and in small groups. |
Expression of epithelial growth factor receptor (EGFR), c-erbB2, Int-2, transforming growth factor-alpha (TGF-α), and human stomach cancer transforming factor (HST-1) has prognostic significance for SCC. EGFR overexpression occurs in 40.65% to 71% of esophageal SCC (109) (Fig. 3.18) and correlates with nodal metastases and depth of invasion. Immunoreactivity for TGF-α in esophageal SCC ranges from 35% to 78% (109,110) and associates with lower survival than seen in TGF-α–negative cancers (p <0.01). Survival is poorest with cancers that overexpress both EGFR and TGF-α (111). Amplification of HST-1 affects approximately 50% of SCCs, associating with hematogenous spread (112). Expression of vascular endothelial growth factor (VEGF), a selective mitogen for endothelial cells, correlates with microvessel density adjacent to the tumor. Shortened survival has been observed in VEGF-positive esophageal SCCs in most studies (113), but not all (114).
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree