Varicocele is defined as an excessive dilation of the pampiniform plexus. The association between varicocele and infertility has been well-established as evidenced by negative effects on spermatogenesis. Accumulating evidence now suggests that varicocele presents a pantesticular insult, with resultant impairment of Leydig cell function. The presence of a varicocele has been linked to lower serum testosterone levels and varicocelectomy may reverse some of the adverse effects on androgen production. In this review, the evidence linking varicoceles to impaired steroidogenesis and which cohorts of men may benefit most from varicocele repair are discussed.
Key points
- •
A multitude of studies suggests an adverse effect of varicocele on Leydig cell function.
- •
Men with lower preoperative serum testosterone levels have greater improvements in postvaricocelectomy testosterone levels as compared with eugonadal men.
- •
The pathophysiology of varicocele-mediated hypogonadism is poorly understood and remains an area of continued investigation.
Introduction
A varicocele is an aberrant dilation of the pampiniform plexus, the network of veins draining the testis. It is a common entity with a prevalence of 10% to 20% in the general population. Most varicoceles are asymptomatic and have an inconsequential impact on the individual’s testicular function. However, a small subset of men will present with infertility, orchialgia, or ipsilateral testicular hypotrophy, which serve as common indications for varicocelectomy.
Traditionally, varicocele has been characterized by its impact on spermatogenesis via local effects on Sertoli and germ cells. It has become more evident, however, that varicocele presents a pantesticular insult. Leydig cell dysfunction is now a recognized potential consequence of varicocele and appears to be a reversible phenomenon with varicocelectomy. Multiple mechanisms for decreased androgen production have been proposed, likely reflecting a multifactorial process. Nevertheless, the pathophysiology of varicocele-mediated Leydig cell dysfunction, as with the cause of the varicocele’s link to subfertility, remains an area of ongoing research.
Introduction
A varicocele is an aberrant dilation of the pampiniform plexus, the network of veins draining the testis. It is a common entity with a prevalence of 10% to 20% in the general population. Most varicoceles are asymptomatic and have an inconsequential impact on the individual’s testicular function. However, a small subset of men will present with infertility, orchialgia, or ipsilateral testicular hypotrophy, which serve as common indications for varicocelectomy.
Traditionally, varicocele has been characterized by its impact on spermatogenesis via local effects on Sertoli and germ cells. It has become more evident, however, that varicocele presents a pantesticular insult. Leydig cell dysfunction is now a recognized potential consequence of varicocele and appears to be a reversible phenomenon with varicocelectomy. Multiple mechanisms for decreased androgen production have been proposed, likely reflecting a multifactorial process. Nevertheless, the pathophysiology of varicocele-mediated Leydig cell dysfunction, as with the cause of the varicocele’s link to subfertility, remains an area of ongoing research.
Clinical data
Early Evidence
Initial reports exploring the possible impact of varicocele on testosterone production were limited by retrospective design, small cohorts, and selection bias. In addition, certain subsets of men will have worse Leydig cell function than others, an issue that may have limited studies that had permissive inclusion criteria. Comhaire and Vermeulen published one of the earliest reports documenting normalization of total testosterone levels in men undergoing varicocelectomy. In their small cohort of 33 men presenting with a clinical varicocele, 10 of the men had low serum testosterone levels (mean <400 ng/dL) and concomitant erectile dysfunction, both of which improved after varicocele repair. This initial account precipitated several other analyses of cohorts of men who presented with infertility and concurrent varicocele. In 1978, Rodriguez-Rigau and colleagues expanded on Comhaire’s work and analyzed a group with palpable varicoceles who also underwent testicular biopsy as part of their fertility evaluation. All subjects had serum testosterone levels in the normal range, albeit subjects with bilateral varicoceles typically had lower levels than those with unilateral varicoceles. Histopathologic assessment of the biopsy samples revealed diminished Leydig cell counts that were especially pronounced in men with concomitant oligospermia. In addition, they identified an abnormal testosterone-to-luteinizing hormone (LH) ratio in men with worse spermatogenesis. From these data, they postulated that varicocele affects all functions and cell lines of the testis.
Multiple small series followed that refuted the concept that varicoceles result in decreased testosterone synthesis. A study by Pirke and colleagues found normal testosterone levels among 21 subjects who presented with varicocele, a result mirrored by Weiss and colleagues, who reported on a cohort of 16 men with accompanying hypospermatogenesis. A contemporaneous analysis by Pasqualini and colleagues also documented normal testosterone levels in a group of 17 patients; however, their data did demonstrate elevated LH levels, leading to the conclusion that men with varicoceles can have normal androgen levels via compensated LH production.
These early conflicting accounts were offset by progressively larger cohorts that offered higher-quality evidence. In 1984, Ando and colleagues published their account of 108 infertile men with varicocele compared against 46 infertile men without varicocele. Those men with varicocele had significantly lower testosterone levels regardless of degree of oligospermia. The group also documented worse testosterone concentrations for those men who had their varicoceles for longer lengths of time. This finding suggested that varicocele imposes progressive negative impacts on both spermatogenesis and Leydig cell function. Subsequently, in 1995, Su and colleagues reviewed their experience in men undergoing varicocelectomy. In a group of 53 patients, they found a statistically significant increase of serum testosterone from a mean preoperative level of 319 ng/dL to a postoperative value of 409 ng/dL. Their analysis also demonstrated an inverse relationship between preoperative testosterone concentration and anticipated postoperative testosterone increase. This finding raised the possibility that some men, especially those with poorer preoperative Leydig cell function, may disproportionately be affected by their varicocele and may have meaningful improvements in testosterone after varicocelectomy.
Contemporary Evidence
The work of Su and colleagues was further bolstered by multiple studies that documented improved testosterone levels following varicocele repair ( Table 1 ). Cayan and colleagues followed 78 men who underwent varicocelectomy, quoting an improvement of mean serum testosterone from 563 to 837 ng/dL. In a similar cohort, Gat and colleagues found significant improvements of total and free testosterone following gonadal vein embolization, suggesting that the positive effect of varicocele repair may not be sensitive to mode of treatment.
| First Author, Year | Study Design | Number Treated for Varicocele | Baseline Testosterone (ng/dL) | Postoperative Testosterone (ng/dL) | Change (ng/dL) | P Value |
|---|---|---|---|---|---|---|
| Shabana et al, 2015 | Prospective | 123 | 385 | 447 | 62 | .0001 |
| Ahmed et al, 2015 | Prospective | 73 | 331 | 357 | 26 | .001 |
| Abdel-Meguid et al, 2014 | Prospective | 66 | 347 | 392 | 45 | .0001 |
| Hsiao et al, 2013 | Retrospective | 78 | 308 | 417 | 109 | .0001 |
| Hsiao et al, 2011 | Retrospective | — | — | — | — | — |
| Age <30 | 31 | NA | NA | 93 | .03 | |
| Age 30–39 | 55 | NA | NA | 59 | .02 | |
| Age >40 | 28 | NA | NA | 73 | .001 | |
| Sathya Srini & Belur Veerachari, 2011 | Prospective | 100 | 177 | 301 | 124 | .001 |
| Tanrikut et al, 2011 | Retrospective | 200 | 358 | 454 | 96 | .001 |
| Zohdy et al, 2011 | Prospective | 103 | 379 | 450 | 71 | .0001 |
| Resorlu et al, 2010 | Retrospective | — | — | — | — | — |
| Age 18–25 | 35 | 275 | 297 | 22 | >.05 | |
| Age 26–35 | 43 | 290 | 306 | 16 | >.05 | |
| Age >36 | 18 | 274 | 291 | 17 | >.05 | |
| Rodriguez-Peña et al, 2009 | Retrospective | 202 | 648 | 709 | 61 | >.05 |
| Ozden et al, 2008 | Prospective | 30 | 660 | 720 | 60 | .1 |
| Di Bisceglie et al, 2007 | Retrospective | 38 | 650 | 660 | 10 | .9 |
| Hurtado de Catalfo et al, 2007 | Retrospective | 36 | 298 | 382 | 84 | NA |
| Gat et al, 2004 | Retrospective | 83 | 348 | 497 | 149 | .001 |
| Fujisawa et al, 2001 | Retrospective | 52 | 460 | 470 | 10 | >.05 |
| Pierik et al, 2001 | Retrospective | 30 | 542 | 571 | 29 | >.05 |
| Cayan et al, 1999 | Retrospective | 78 | 563 | 837 | 274 | .01 |
| Su et al, 1995 | Retrospective | 53 | 319 | 409 | 90 | .001 |
Despite these compelling data, many investigators continued to find insignificant improvements of testosterone following varicocelectomy. Rodriguez-Peña and colleagues reported a group of 202 patients with grade II or III varicoceles. Their results demonstrated a mean testosterone increase of 61 ng/dL, although the finding did not reach statistical significance. In another large series by Al-Ali colleagues in which 1111 men had presented for infertility evaluation, the presence of grade III varicoceles was actually associated with higher testosterone levels. These conflicting reports were ultimately contextualized by Hsiao and colleagues in 2011. In this series, 106 men underwent hormonal evaluation before and after varicocelectomy similar to previous study designs. However, Hsiao and colleagues stratified their cohort into men with eugonadal testosterone levels and biochemical hypogonadism (≥ or <400 ng/dL, respectively). As was the case with Su’s initial finding in 1996, men with lower initial serum testosterone experienced far greater increases in their androgen levels as opposed to the eugonadal individuals. Many of the studies that did not find significant improvements of serum testosterone following varicocele repair had cohorts characterized by normal preoperative testosterone levels (ie, greater than 400 ng/dL). For instance, the study subjects of Rodriguez-Peña and colleagues had a mean preoperative testosterone level of 648 ng/dL. The cohort of Pierik and colleagues was also eugonadal in 90% of the cases.
Several studies have corroborated Hsiao’s finding. Tanrikut and colleagues examined a large series of 325 men who had undergone varicocelectomy for infertility and contrasted them against 510 men who presented for vasectomy. The varicocele group had significantly lower serum testosterone levels than control subjects. Of the 200 men in whom both preoperative and postoperative hormonal profiles were available, a mean testosterone increase of 96 ng/dL was demonstrated. They found that 79% of the men with initial testosterone levels less than 300 ng/dL had normal testosterone concentrations following varicocelectomy. A similar study by Sathya Srini and Belur Veerachari included only those infertile men with varicoceles and preoperative testosterone levels less than 280 ng/dL. Half of their cohort underwent varicocele repair, whereas the other half chose to proceed directly to assisted reproduction. The surgical group had postoperative normalization of their testosterone levels in 78% of cases, whereas only 16% of control subjects had normalization over the same time span. The most dramatic results that expanded on the observations of Su and colleagues and Hsiao and colleagues were published by Abdel-Meguid and colleagues in 2014. In this well-powered study, only those men with pre-existing biochemical hypogonadism (<300 ng/dL) had a significant increase of testosterone level following varicocele repair. This subgroup of patients had a mean testosterone increment of 93.7 ng/dL as opposed to 8.6 ng/dL in eugonadal men.
The quality of evidence linking varicocelectomy to increased androgen production has continued to improve with time. A summative meta-analysis by Li and colleagues incorporated 9 studies with a total of 814 patients. The pooled data yielded a mean testosterone increase of 97.5 ng/dL following varicocele repair. More recently, investigators have used prospective study designs. Ahmed and colleagues enrolled 129 patients and followed them to 6 months after varicocelectomy. The mean testosterone concentration increased from 331 to 357 ng/dL. In 2015, Shabana and colleagues published a similar prospective study with a cohort of 123 patients noting mean prevaricocelectomy and postvaricocelectomy values of 385 and 447 ng/dL, respectively. These 2 studies complemented other prospective trials that demonstrated similar results .
The preponderance of evidence supports the link between varicocele and diminished Leydig cell function in man. Unfortunately, one must rely on a set of publications that may be flawed by selection bias, given the impracticality of conducting a randomized trial in patients who often present with infertility and desire varicocelectomy. Even when viewed from this lens, one cannot ignore the number of well-fashioned studies that include relatively large numbers of subjects. Although a great deal of work has thus established the link between varicocele and decreased androgen production, the pathophysiologic principles that result in this association have yet to be identified.
Pathophysiology of Leydig cell dysfunction
Human Studies
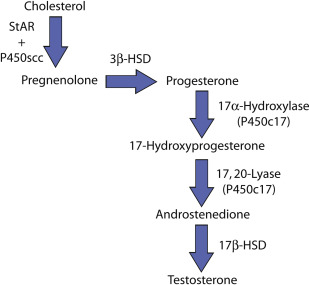
The mechanisms proposed to explain how varicoceles may adversely affect Leydig cells closely parallel the classic theories of varicocele-mediated infertility. It is likely that the Leydig cell reacts similarly to Sertoli and germ cells when exposed to hypoxia, hyperthermia, gonadal/adrenal toxins, and oxidative stress, all of which may be derived from impaired venous drainage. Some investigators have even proposed that impaired testosterone secretion is causal to subsequent hypospermatogenesis. Others have submitted evidence that deficient spermatogenesis and Leydig cell dysfunction are independent processes that share common upstream causal events. Although both viewpoints seem to be at odds, as with most biologic systems, it is likely that decreased testosterone synthesis and impaired sperm production share both a direct causal link and common precursor mechanisms.
Early human studies concentrated on the hypothalamic-pituitary-testis (HPT) axis in men with concomitant varicocele and subfertility. Hudson and colleagues used a bolus of gonadotropin-releasing hormone (GnRH) to study the HPT axis in men with and without varicocele. They repeated the protocol in subjects who underwent varicocele repair. Their data demonstrated that men with varicoceles typically have an excessive release of LH in response to the GnRH bolus as compared with normal individuals. In addition, 63% of men who had varicocelectomy experienced normalization of their serum testosterone response to the GnRH stimulation. The investigators concluded that varicoceles result in a global testicular defect as evidenced by derangement of the HPT axis, which may be reversible in some men who undergo varicocele repair. The early work by Hudson was further developed by Ando and colleagues, who followed an expanded hormonal profile within peripheral and spermatic cord veins. These investigators also used a GnRH bolus to probe the HPT axis of subjects with varicocele. Their results corroborated the tendency of diminished testosterone secretion in varicocele-afflicted men when compared with controls. The broader analysis of the steroidogenic pathway enabled Ando and his colleagues to evaluate for potential enzymatic block or dysfunction. In men with varicocele, they demonstrated excessive amounts of 17-hydroxyprogesterone and a markedly elevated 17-hydroxyprogesterone:testosterone ratio ( Fig. 1 ). These data indicate a possible defect at the level of 17,20-lyase and, to a lesser degree, 17α-hydroxylase. It has been proposed that these enzymes are particularly sensitive to hyperthermia, a known consequence of varicocele formation. A subsequent study by Osuna and colleagues confirmed similar endocrine results in an adolescent cohort with varicocele and preserved total testosterone.