CHAPTER 48 Testicular and Paratesticular Tumors
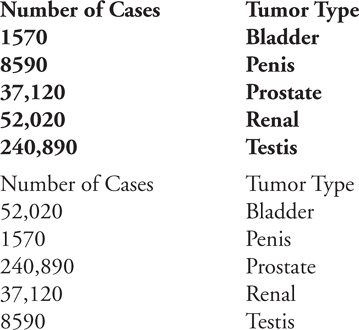
![]() The following are the number of new cancer cases for several urinary cancers in men in the United States in 2011. Match the number of cases to the tumor type.
The following are the number of new cancer cases for several urinary cancers in men in the United States in 2011. Match the number of cases to the tumor type.
![]() The reported incidence of testicular cancer varies greatly between countries. Within the United States, what, if any, is the effect of race on testicular cancer incidence?
The reported incidence of testicular cancer varies greatly between countries. Within the United States, what, if any, is the effect of race on testicular cancer incidence?
The incidence of testicular cancer in the United States in non-Hispanic whites is 5 times higher than the incidence in African-Americans, 4 times higher than the incidence in Asians, and 78% higher than in Hispanics.
![]() What are the most common genetic alterations found in testis cancer?
What are the most common genetic alterations found in testis cancer?
An isochromosome of the short arm of chromosome 12, i (12p), is a relatively frequent finding in germ cell tumors. Tumors not containing i (12p) generally have additional genetic material located on the short arm of chromosome 12. Isochromosome 12p is found in up to 80% of germ cell tumors. Interestingly, neither gain nor amplification of 12p material is commonly seen in intratubular germ cell neoplasia (although many other germ cell genomic changes are already present) suggesting that gain of genetic material from 12p is associated with the progression from intratubular germ cell neoplasia to invasive disease.
![]() A 2-year-old child undergoes a right orchiopexy for a high inguinal undescended testicle. What is his risk of developing testis cancer in that testicle at some point in the future?
A 2-year-old child undergoes a right orchiopexy for a high inguinal undescended testicle. What is his risk of developing testis cancer in that testicle at some point in the future?
Patients with cryptorchidism have a 2.7 to 8 times increased risk of developing testicular cancer. The lifetime probability of developing testis cancer is therefore between 0.6% and 1.6%.
![]() A 2-year-old child with a high inguinal undescended testicle has an orchiopexy. To what degree will the orchiopexy affect his future risk of developing testis cancer and what effect, if any, does puberty have on this risk?
A 2-year-old child with a high inguinal undescended testicle has an orchiopexy. To what degree will the orchiopexy affect his future risk of developing testis cancer and what effect, if any, does puberty have on this risk?
There is controversy about the degree to which orchiopexy decreases the risk of testis cancer. Orchiopexy certainly allows for easier examination of the affected testicle and potentially earlier detection. A large population-based study showed children who had an orchiopexy prior to age 13 had an increased relative risk of testis cancer of 2.2, whereas those who had an orchiopexy after age 13 had an increased relative risk of 5.4 when compared to the general population.
![]() A 2-year-old child undergoes a right orchiopexy for a high inguinal undescended testicle. He is at increased risk of developing testis cancer in the right testicle. Is he at greater risk than the general population of developing a tumor in his normal descended left testicle?
A 2-year-old child undergoes a right orchiopexy for a high inguinal undescended testicle. He is at increased risk of developing testis cancer in the right testicle. Is he at greater risk than the general population of developing a tumor in his normal descended left testicle?
Although it has been previously thought that the normal descended testicle contralateral to an undescended testicle has an increased risk of developing cancer, this is now being challenged. Although a case–control study from the Danish Cancer Registry found an increased risk (HR of 3.6), other studies have not consistently confirmed these findings. Combined data from numerous large case series now suggest that the incidence may in fact be similar to that of the general population.
![]() What is/are the peak age group(s) for the development of Leydig cell tumor and how do these tumors present?
What is/are the peak age group(s) for the development of Leydig cell tumor and how do these tumors present?
Leydig cell tumors have a bimodal distribution, with an initial peak in the prepubertal age group, 4 to 10 and then a second peak between the ages of 30 and 60. They present most often with gynecomastia, precocious puberty, or breast tenderness.
![]() β-hCG is a tumor marker that is commonly measured in patients with testis cancer. What cells produce β-hCG? What percentage of patients will demonstrate an elevated β-hCG with pure seminoma and nonseminomatous germ cell tumor (NSGCT)?
β-hCG is a tumor marker that is commonly measured in patients with testis cancer. What cells produce β-hCG? What percentage of patients will demonstrate an elevated β-hCG with pure seminoma and nonseminomatous germ cell tumor (NSGCT)?
β-hCG is produced by syncytiotrophoblastic cells. β-hCG is elevated in approximately 15% of patients with pure seminoma and 40% to 60% of those with a NSGCT.
![]() Alpha-fetoprotein (AFP) is a tumor marker that is commonly measured in patients with testis cancer. What percentages of patients with pure seminoma have an elevated AFP and what percentage of patients with NSGCT have elevated AFP levels?
Alpha-fetoprotein (AFP) is a tumor marker that is commonly measured in patients with testis cancer. What percentages of patients with pure seminoma have an elevated AFP and what percentage of patients with NSGCT have elevated AFP levels?
AFP is not elevated in patients with pure seminoma. AFP is elevated in 50% to 70% of patients with NSGCT.
![]() A 25-year-old has a right orchiectomy for a NSGCT. Prior to orchiectomy, his AFP was 124 and his β-hCG was 88. What is the half-life of AFP and β-hCG? Assuming that he has no metastatic disease, when should his AFP and β-hCG return to normal?
A 25-year-old has a right orchiectomy for a NSGCT. Prior to orchiectomy, his AFP was 124 and his β-hCG was 88. What is the half-life of AFP and β-hCG? Assuming that he has no metastatic disease, when should his AFP and β-hCG return to normal?
The metabolic half-life of AFP is 5 to 7 days. Thus, using conservative estimates, the AFP should be normal (<9) in about 4 weeks. The serum half-life of β-hCG is 24 to 36 hours. Thus, using conservative estimates, the β-hCG should be normal (<2) in about 9 days.
![]() A family practitioner refers a 3-month-old boy to you for evaluation of a left testis mass. He was concerned about a testis tumor and sent tumor markers. AFP was 57 and β-hCG was <2. On examination, the infant has a swollen left testis that is twice the size of the right testis. The epididymis cannot readily be distinguished from the testis. What is the next step in the evaluation and how likely is this child to have testicular cancer?
A family practitioner refers a 3-month-old boy to you for evaluation of a left testis mass. He was concerned about a testis tumor and sent tumor markers. AFP was 57 and β-hCG was <2. On examination, the infant has a swollen left testis that is twice the size of the right testis. The epididymis cannot readily be distinguished from the testis. What is the next step in the evaluation and how likely is this child to have testicular cancer?
The next step in evaluation is a testicular ultrasound. The child is very unlikely to have testis cancer. The physical examination is much more suggestive of epididymitis or a missed torsion. Testicular cancer is unusual in infants. The most common testicular cancer would be a yolk sac tumor where the peak incidence is 2 years of age. AFP is produced by the fetus and is measurable at very high levels from the 12th week of gestation. The level gradually falls thereafter and reaches normal levels by the end of the first year of life.
![]() A 22-year-old college student was playing a vigorous game of touch football. He does not specifically remember being hit in the genital area but following the game he has an aching pain in his right testicle. He performs a testicular self-examination and feels a testicular mass. Your examination confirms that the right testicle is larger than the left with some thickening at the lower pole. How should the testicle be evaluated?
A 22-year-old college student was playing a vigorous game of touch football. He does not specifically remember being hit in the genital area but following the game he has an aching pain in his right testicle. He performs a testicular self-examination and feels a testicular mass. Your examination confirms that the right testicle is larger than the left with some thickening at the lower pole. How should the testicle be evaluated?
Scrotal ultrasound should be the next step in the evaluation. Many testis tumors are noticed after only minor trauma, but in this case, the diagnosis is uncertain. Therefore, the initial evaluation should be the inexpensive, noninvasive, and sensitive screening study of a scrotal ultrasound.
![]() A 24-year-old has a testicular ultrasound for evaluation of a dragging sensation in his right testicle. He is referred to see you as the ultrasound showed “bilateral microlithiasis.” The patient has no other complaints or problems. His discomfort is improving and has been attributed to a sports injury. His physical examination is normal with no testicular masses. The ultrasound is a good-quality study and shows approximately 15 to 20 small (<2 mm) microcalcifications distributed throughout the body of both testes. What is the risk of testicular cancer and what should be the next step in his evaluation? What is the recommended follow-up?
A 24-year-old has a testicular ultrasound for evaluation of a dragging sensation in his right testicle. He is referred to see you as the ultrasound showed “bilateral microlithiasis.” The patient has no other complaints or problems. His discomfort is improving and has been attributed to a sports injury. His physical examination is normal with no testicular masses. The ultrasound is a good-quality study and shows approximately 15 to 20 small (<2 mm) microcalcifications distributed throughout the body of both testes. What is the risk of testicular cancer and what should be the next step in his evaluation? What is the recommended follow-up?
Instruct the patient to perform regular testis self-examination and seek prompt evaluation if he notices a mass. Large studies of army recruits have demonstrated that testicular microlithiasis is not associated with a substantially increased risk of subsequent testis cancer development. Thus, no further evaluation is necessary, short of teaching the patient to perform testicular self-examination.
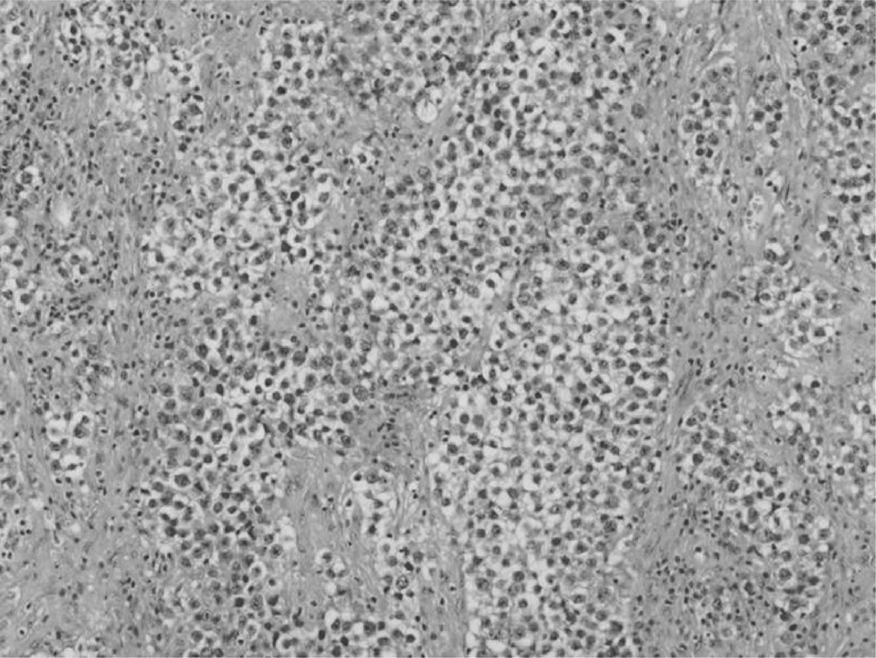
![]() A 62-year-old man has gradual painless swelling of his left testicle. Physical examination is noticeable for diffuse enlargement and firmness of the left testicle. The right testis is normal. The remainder of his physical examination is normal. A left radical orchiectomy is performed. A low-power photomicrograph is shown below (Fig. 1). What is the diagnosis?
A 62-year-old man has gradual painless swelling of his left testicle. Physical examination is noticeable for diffuse enlargement and firmness of the left testicle. The right testis is normal. The remainder of his physical examination is normal. A left radical orchiectomy is performed. A low-power photomicrograph is shown below (Fig. 1). What is the diagnosis?
Figure 1.
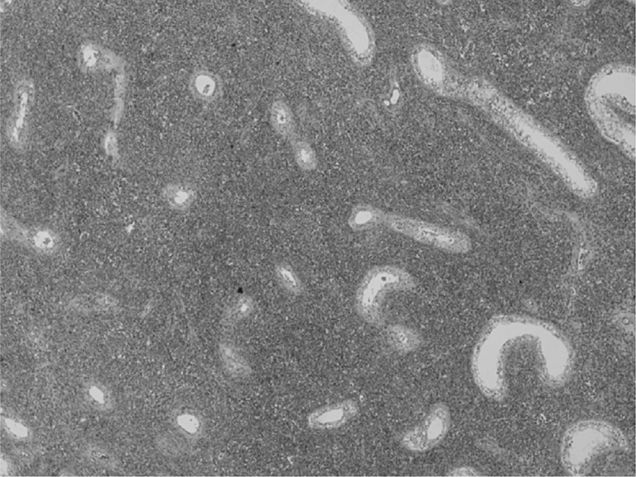
Lymphoma. It is the most common testicular tumor in men older than 60 years. The photomicrograph shows tubules surrounded by monotonous blue staining cells, an appearance that is characteristic for testicular infiltration by lymphoma.
![]() What is the rationale for performing an inguinal approach to orchiectomy as part of the treatment of testicular cancer when a scrotal approach is appropriate when performing an orchiectomy as part of the treatment for prostate cancer?
What is the rationale for performing an inguinal approach to orchiectomy as part of the treatment of testicular cancer when a scrotal approach is appropriate when performing an orchiectomy as part of the treatment for prostate cancer?
When operating on testicular cancer, a trans-scrotal approach is contraindicated as it may alter the lymphatic drainage of the testis, increasing the risk of local recurrence and pelvic or inguinal lymph node metastasis. There are no such concerns in prostate cancer as in that scenario the procedure is being done solely for the removal of the androgen producing components of the testes.
![]() A 59-year-old man noticed a mass at the upper pole of the right testicle. He is otherwise healthy and asymptomatic. β-hCG is 110 (normal <2). AFP is 256 (normal <9). His ultrasound is shown below (Fig. 2). He undergoes a right radical orchiectomy. What is the most likely diagnosis?
A 59-year-old man noticed a mass at the upper pole of the right testicle. He is otherwise healthy and asymptomatic. β-hCG is 110 (normal <2). AFP is 256 (normal <9). His ultrasound is shown below (Fig. 2). He undergoes a right radical orchiectomy. What is the most likely diagnosis?
Figure 2.
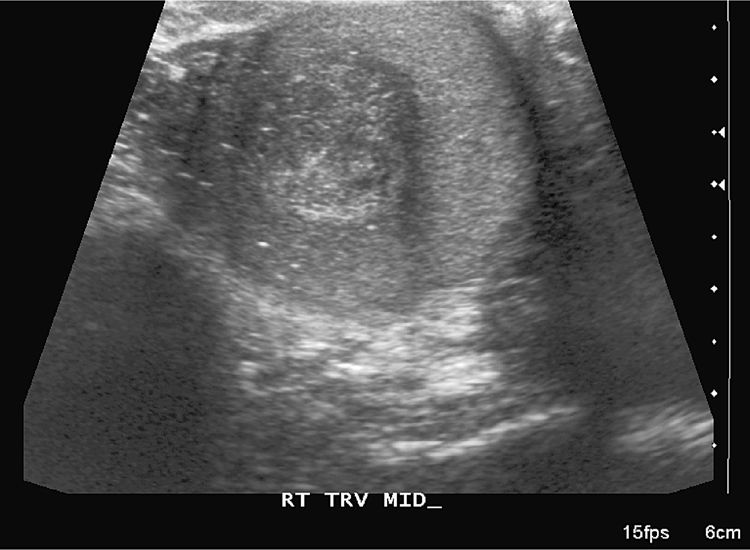
Mixed germ cell tumor. A seminoma would be a more common tumor in this age group. However, the presence of an elevated AFP excludes this diagnosis. The presence of elevated tumor markers essentially rules out a lymphoma and a Leydig cell tumor.
![]() A 35-year-old man has a right testis biopsy as part of an evaluation for infertility and is found to have testicular intraepithelial neoplasia (TIN) (or intratubular germ cell neoplasia). Prior to the testis biopsy, he had a small right testis but no masses were palpable. A right testis U/S showed a decrease in testicular volume with normal echo texture throughout. As a child, he had a left undescended testicle that was removed at age 8. He is otherwise healthy. His physical examination is normal. He is azoospermic. His FSH is elevated at 3 times normal. Serum testosterone is normal. What are the treatment options and what is his best option to both minimize his risk of developing testis cancer and maintain as much physiological function as possible?
A 35-year-old man has a right testis biopsy as part of an evaluation for infertility and is found to have testicular intraepithelial neoplasia (TIN) (or intratubular germ cell neoplasia). Prior to the testis biopsy, he had a small right testis but no masses were palpable. A right testis U/S showed a decrease in testicular volume with normal echo texture throughout. As a child, he had a left undescended testicle that was removed at age 8. He is otherwise healthy. His physical examination is normal. He is azoospermic. His FSH is elevated at 3 times normal. Serum testosterone is normal. What are the treatment options and what is his best option to both minimize his risk of developing testis cancer and maintain as much physiological function as possible?
The best option to minimize testis cancer risk and preserve as much physiological function as possible is low-dose radiation to the testicle (16-20 Gy). The patient is almost certainly infertile given his elevated FSH and azoospermia. Therefore, preservation of endocrine function and eradication of TIN are the primary goals of therapy; 16 to 20 Gy of irradiation has been shown to effectively achieve this goal in European studies. Chemotherapy can also eradicate TIN. However, the optimal schedule is not known and it is less reliable than radiation. Observation is an option, but the patient is quite likely to develop a germ cell tumor at a future date. Radical orchiectomy will cure the cancer but not preserve his endocrine function.
![]() A 32-year-old man undergoes a right orchiectomy for a stage I NSGCT and enters a surveillance protocol. At the end of 1 year, he is asymptomatic, all radiologic and laboratory studies are negative. He asks you what his chances are of developing a tumor in the opposite testicle.
A 32-year-old man undergoes a right orchiectomy for a stage I NSGCT and enters a surveillance protocol. At the end of 1 year, he is asymptomatic, all radiologic and laboratory studies are negative. He asks you what his chances are of developing a tumor in the opposite testicle.
Approximately 2% to 4% of patients develop a contralateral germ cell tumor which is a 20- to 40-fold increased risk compared to the general population. For this reason, it is important to teach patients with testicular tumors to perform regular contralateral testicular self-examination.
![]() A 28-year-old man who, 2 years previously, had a left orchiectomy following a traumatic testis injury presents with a 2-cm right lower pole testicular mass. Tumor markers are negative. An ultrasound demonstrates a solid mass located on the anterior surface of the lower pole of the right testis. The remainder of the testis is normal. The patient is recently married and is interested in having children in the future. What are the management options?
A 28-year-old man who, 2 years previously, had a left orchiectomy following a traumatic testis injury presents with a 2-cm right lower pole testicular mass. Tumor markers are negative. An ultrasound demonstrates a solid mass located on the anterior surface of the lower pole of the right testis. The remainder of the testis is normal. The patient is recently married and is interested in having children in the future. What are the management options?
There are 3 management options:
1. Radical orchiectomy, which is associated with significant permanent endocrine abnormalities and sterility but assures local control.
2. Partial orchiectomy followed by low-dose radiation (16-20 Gy), which is associated with sterility but preserves endocrine function and is associated with a low incidence of local recurrence.
3. Partial orchiectomy alone, which preserves endocrine function and may preserve fertility but is associated with a higher risk of local failure. As this patient has a favorably located tumor, partial orchiectomy with negative margins preceded by sperm banking would be a good therapeutic choice, if the patient desires to father a child. He should subsequently be advised to have delayed low-dose radiation after he completes his family.
![]() A 22-year-old man has a right orchiectomy for a pT2 mixed germ cell tumor. Prior to orchiectomy, his β-hCG was 256, AFP was 58, and lactic dehydrogenase (LDH) was normal. He is asymptomatic and his physical examination is unremarkable. All of the following are imaging options; chest x-ray, CT of chest and abdomen, PET/CT fusion imaging of chest and abdomen. Which is the most appropriate choice?
A 22-year-old man has a right orchiectomy for a pT2 mixed germ cell tumor. Prior to orchiectomy, his β-hCG was 256, AFP was 58, and lactic dehydrogenase (LDH) was normal. He is asymptomatic and his physical examination is unremarkable. All of the following are imaging options; chest x-ray, CT of chest and abdomen, PET/CT fusion imaging of chest and abdomen. Which is the most appropriate choice?
CT of the chest and abdomen: The most common sites of disease in a patient with a newly diagnosed NSGCT are the retroperitoneal lymph nodes and the lungs. These areas should be routinely imaged. In the past, investigators have argued that a chest x-ray is sufficient for lung imaging in the absence of disease in the retroperitoneum. However, a chest CT has a much higher sensitivity than a chest x-ray and is almost certainly a better choice. A PET CT fusion study is much more expensive and in a prospective study has not been shown to provide reliable additional information in patients with NSGCT.
![]() A 19-year-old man has a right orchiectomy for a pT2 NSGCT. His tumor markers, which were previously elevated, have returned to normal as predicted by their half-lives. His CT scan of the chest and abdomen are normal. He has seen a medical oncologist, who, after reviewing the pathology report, told him that if he had an retroperitoneal lymph node dissection (RPLND) the chances that microscopic disease would be found in his lymph nodes were approximately 40% to 60%. What additional information in the pathology report allowed the medical oncologist to draw this conclusion?
A 19-year-old man has a right orchiectomy for a pT2 NSGCT. His tumor markers, which were previously elevated, have returned to normal as predicted by their half-lives. His CT scan of the chest and abdomen are normal. He has seen a medical oncologist, who, after reviewing the pathology report, told him that if he had an retroperitoneal lymph node dissection (RPLND) the chances that microscopic disease would be found in his lymph nodes were approximately 40% to 60%. What additional information in the pathology report allowed the medical oncologist to draw this conclusion?
The presence of vascular invasion and the percentage of embryonal cancer. The combination of vascular invasion and a high percentage (>40%) of embryonal cancer in the primary tumor is associated with an elevated risk of finding disease in the retroperitoneum at RPLND.
![]() A 22-year-old single student has a newly diagnosed International Germ Cell Cancer Consensus (IGCCC) low risk metastatic testis cancer. He has seen a medical oncologist and is fit for and understands the risks of chemotherapy. However, prior to initiating chemotherapy, he should consider what additional precautionary procedure?
A 22-year-old single student has a newly diagnosed International Germ Cell Cancer Consensus (IGCCC) low risk metastatic testis cancer. He has seen a medical oncologist and is fit for and understands the risks of chemotherapy. However, prior to initiating chemotherapy, he should consider what additional precautionary procedure?
Sperm banking. Treatment of testis cancer has the potential to lead to azoospermia. Therefore, all patients should be encouraged to consider sperm banking prior to initiating therapy. Between 40% and 60% of patients with testicular cancer are hypofertile at diagnosis with sperm counts returning to normal in 75% of patients following orchiectomy. With newer reproductive technologies, fertility is likely to be possible for more than 80% of patients. Sperm banking is a sensible precaution as the sperm can be discarded after 2 to 3 years if the patient remains disease-free and the sperm count reverts to normal.
![]() A 30-year-old man presents with a left testicular mass. He undergoes a left orchiectomy. Pathology shows a 4-cm embryonal cancer invading the tunica albuginea. Vascular invasion is noted. Metastatic workup consists of an abdominal MRI scan (Fig. 3). Chest CT scan shows 5 0.5 cm to 2 cm lung nodules. LDH is 3 times normal. β-hCG is 3000. AFP is 1500. What stage is the patient according to the 7th edition (2010) of the Manual of American Joint Commission on Cancer TNM staging system?
A 30-year-old man presents with a left testicular mass. He undergoes a left orchiectomy. Pathology shows a 4-cm embryonal cancer invading the tunica albuginea. Vascular invasion is noted. Metastatic workup consists of an abdominal MRI scan (Fig. 3). Chest CT scan shows 5 0.5 cm to 2 cm lung nodules. LDH is 3 times normal. β-hCG is 3000. AFP is 1500. What stage is the patient according to the 7th edition (2010) of the Manual of American Joint Commission on Cancer TNM staging system?
Figure 3.
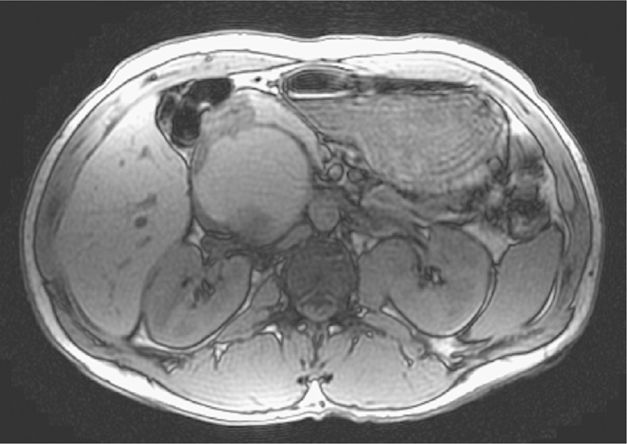
MRI shows a 9-cm intra-aortocaval nodal mass. The patient has a T2N3S2M1a, stage IIIB tumor.
![]() A 35-year-old man is diagnosed with a T1N0M0S1 pure seminoma. His β-hCG was 56 at diagnosis and 14 days later is <2. His CT scan of the chest, abdomen, and pelvis are normal. What is his risk of recurrence if he has no further treatment? How does this risk change if he elects to receive 20 to 25 Gy of radiation therapy to the standard retroperitoneal field?
A 35-year-old man is diagnosed with a T1N0M0S1 pure seminoma. His β-hCG was 56 at diagnosis and 14 days later is <2. His CT scan of the chest, abdomen, and pelvis are normal. What is his risk of recurrence if he has no further treatment? How does this risk change if he elects to receive 20 to 25 Gy of radiation therapy to the standard retroperitoneal field?
The risk of recurrence for a patient with a stage I seminoma on surveillance is approximately 15%. In large studies, the risk of recurrence with adjuvant radiation for stage I seminoma is 1% to 3%.
![]() The same patient as in the previous question is considering either adjuvant radiation or a single dose of carboplatin as adjuvant therapy for his stage I seminoma. Should he choose radiation, what is the recommended field of radiation and why?
The same patient as in the previous question is considering either adjuvant radiation or a single dose of carboplatin as adjuvant therapy for his stage I seminoma. Should he choose radiation, what is the recommended field of radiation and why?
He should be treated with a para-aortic field, alone, rather than a “dog leg” field that had been recommended in the past. A large randomized trial comparing para-aortic radiation to para-aortic and ipsilateral iliac lymph node irradiation found no difference in relapse free survival (96% at 5 years). Acute toxicities were less common and sperm counts higher in the para-aortic only group.
![]() The same patient as in the previous question continues to consider either adjuvant radiation or a single dose of carboplatin as adjuvant therapy for his stage I seminoma. What are the advantages, if any, of single dose of carboplatin when compared to radiation therapy?
The same patient as in the previous question continues to consider either adjuvant radiation or a single dose of carboplatin as adjuvant therapy for his stage I seminoma. What are the advantages, if any, of single dose of carboplatin when compared to radiation therapy?
A large, randomized study demonstrated equivalence of 20 Gy of retroperitoneal irradiation to a single dose of carboplatin with regards to cancer control. Subjects in the carboplatin arm had less of a decrease in their quality of life scores and had a decrease in the frequency of secondary contralateral testis tumors.
![]() A 28-year-old man is diagnosed with an anaplastic seminoma after a left radical orchiectomy. Tumor markers are normal. A CT scan of the chest is normal. A CT scan of the abdomen demonstrated a 2-cm left para-aortic lymph node just below the left renal vein. The patient is asymptomatic and otherwise healthy. What is the recommended treatment? How would this be different if the patient had a pure seminoma as compared to an anaplastic seminoma?
A 28-year-old man is diagnosed with an anaplastic seminoma after a left radical orchiectomy. Tumor markers are normal. A CT scan of the chest is normal. A CT scan of the abdomen demonstrated a 2-cm left para-aortic lymph node just below the left renal vein. The patient is asymptomatic and otherwise healthy. What is the recommended treatment? How would this be different if the patient had a pure seminoma as compared to an anaplastic seminoma?
The recommended treatment is 30 to 36 Gy of retroperitoneal radiation to a retroperitoneal and ipsilateral iliac field. There is no evidence that anaplastic seminomas should be treated differently to standard seminomas stage for stage. There is some evidence that seminomas with an anaplastic histology present at a more advanced stage. Standard therapy for a low-volume stage II seminoma is retroperitoneal irradiation.
![]() A 63-year-old man is diagnosed with a spermatocytic seminoma after a left radical orchiectomy. Tumor markers are normal. His CT scan of the chest, abdomen, and pelvis are normal. What is the recommended treatment? How would this be different if the patient had a pure seminoma as compared to a spermatocytic seminoma?
A 63-year-old man is diagnosed with a spermatocytic seminoma after a left radical orchiectomy. Tumor markers are normal. His CT scan of the chest, abdomen, and pelvis are normal. What is the recommended treatment? How would this be different if the patient had a pure seminoma as compared to a spermatocytic seminoma?
The risk of metastatic disease associated with spermatocytic seminoma is extremely low. Thus, surveillance is the recommended course of action. If the patient had a pure seminoma, the risk of recurrence is approximately 15%, thus a discussion of adjuvant radiation or single dose of carboplatin is appropriate.
![]() A 44-year-old man has a right orchiectomy for a 4-cm testis tumor. A representative high power micrograph is shown below (Fig. 4). Preoperative tumor markers were notable for a β-hCG of 200. AFP is normal and LDH is 1.3 times normal. A CT scan of the chest is normal. A CT scan of the abdomen and pelvis shows a large 7-cm mass surrounding the aorta and inferior vena cava, just below the renal vessels. The patient is otherwise healthy and asymptomatic. One week after his orchiectomy, his β-hCG is 108. What is the diagnosis, tumor stage, and recommended therapy?
A 44-year-old man has a right orchiectomy for a 4-cm testis tumor. A representative high power micrograph is shown below (Fig. 4). Preoperative tumor markers were notable for a β-hCG of 200. AFP is normal and LDH is 1.3 times normal. A CT scan of the chest is normal. A CT scan of the abdomen and pelvis shows a large 7-cm mass surrounding the aorta and inferior vena cava, just below the renal vessels. The patient is otherwise healthy and asymptomatic. One week after his orchiectomy, his β-hCG is 108. What is the diagnosis, tumor stage, and recommended therapy?
Figure 4.
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree


