Technical Considerations in the Surgical Management of Presacral Tumors
Najjia Mahmoud
Robert Fry
Presacral tumors, whether benign or malignant, represent a very small proportion of neoplasms encountered in clinical practice. Estimates of incidence vary and are difficult to precisely obtain, but in U.S. adults, patients with retrorectal tumors are thought to present to major referral centers two to six times per year (1). A study from the Mayo clinic reported that retrorectal tumors accounted for one in 40,000 hospital admissions (2).
Presacral masses are classified into categories that tend to reflect the histologically heterogeneous nature of the presacral space itself. These masses may be congenital, neurogenic, inflammatory, osseous, and “miscellaneous” (Table 19.1). Undoubtedly, the most challenging issue for surgeons is the location of these malignancies. The small, confined space defined by the framework of the lower bony pelvis houses nerves, vessels, muscles, and genitourinary and gastrointestinal organs within millimeters of one another. An invasive mass in this area represents special technical challenges and almost always portends the loss of function and form, either from surgery or the neoplasm itself. The most important preoperative decision when treating to cure or palliate is the creation of a multidisciplinary team appropriate for the location of the mass. Clearly, these rare tumors require careful preoperative planning for best short- and long-term outcomes. This chapter serves to categorize these tumors, discuss preoperative planning, and offer technical approaches and insights into surgical extirpation and reconstruction.
Anatomy
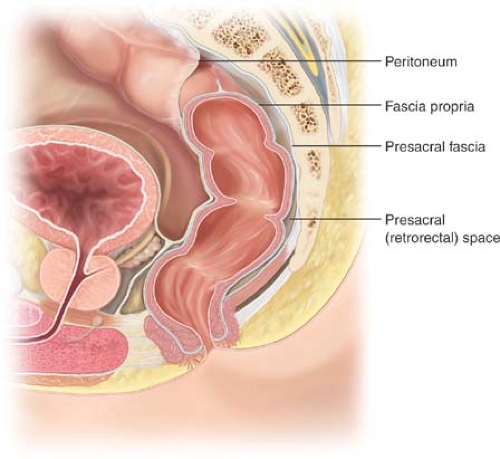
The pelvis is defined by the confines of its bony structure—the sacrum posteriorly, pubic bone anteriorly, and the sacral rami laterally. It is invested by the endopelvic fascia called the “presacral fascia” anterior to the sacrum (Fig. 19.1). Along the presacral fascia run the hypogastric nerves as they branch laterally and anteriorly to coalesce with their parasympathetic counterparts to form the sacral plexus. Laterally, the internal
and external iliac vessels and the ureters run along the retroperitoneum anterior to the iliopsoas muscle and the bony framework of the true pelvis. Retrorectal masses may reside between the fascia propria of the rectum posteriorly and the presacral fascia. The space is actually a potential one, bound by the rectum (and mesorectum) anteriorly, the presacral fascia posteriorly, and the endopelvic fascia (lateral ligaments) laterally. The inferior border is Waldeyer’s fascia overlying the perineal muscles and the superior boundary is the peritoneal reflection. These masses may also arise from neural or osseous elements located posterior to this. The retrorectal space contains elements derived embryologically from the hindgut, notochord, and neuroectoderm; consequently, tumors that arise are heterogeneous.
and external iliac vessels and the ureters run along the retroperitoneum anterior to the iliopsoas muscle and the bony framework of the true pelvis. Retrorectal masses may reside between the fascia propria of the rectum posteriorly and the presacral fascia. The space is actually a potential one, bound by the rectum (and mesorectum) anteriorly, the presacral fascia posteriorly, and the endopelvic fascia (lateral ligaments) laterally. The inferior border is Waldeyer’s fascia overlying the perineal muscles and the superior boundary is the peritoneal reflection. These masses may also arise from neural or osseous elements located posterior to this. The retrorectal space contains elements derived embryologically from the hindgut, notochord, and neuroectoderm; consequently, tumors that arise are heterogeneous.
Table 19.1 Classification of Retrorectal Masses | |||||||||||||||||||||||||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| |||||||||||||||||||||||||||||||||||||||||||||||||||||||
Congenital Lesions
Congenital lesions constitute two-thirds of all lesions found in the retrorectal space and represent the persistence and eventual growth of embryologic elements (3). Developmental cysts, anterior meningoceles, chordomas, rectal duplications and tailgut cysts, and adrenal rest tumors all derive from persistent embryologic elements and are considered congenital in nature. In adults, cystic congenital tumors are typically benign and solid ones are usually malignant.
Developmental Cysts
Developmental cysts constitute two-thirds of the retrorectal tumors encountered in clinical practice and are almost always benign (4). They have a prominent female preponderance of approximately 5:1 in most series (4). They can arise from any embryonic cell layer. Epidermoid, dermoid, or teratoma tumors are the three most common entities and are comprised of one, two, and three embryologically distinct cell layer origins, respectively (Fig. 19.2). Tailgut cysts are included in the developmental cyst group, although they are quite rare, and are derived from mesodermal tissue of the embryonic gastrointestinal tract. Tailgut cysts are considered retrorectal cystic hamartomas and result from failure of regression of the embryonic tail (5,6). Rectal duplication cysts are similarly rare and frequently harbor a secretory gut epithelial lining as well as all of the layers of the rectal wall. Like intestinal duplication cysts elsewhere, they may share a common wall with the true rectum. Dermoid and epidermoid cysts often contain dermal elements and are commonly filled with a sebaceous-like material secreted from glands within the cyst wall. Dermoid cysts are the most commonly encountered retrorectal mass in clinical practice, and a simple posterior juxtasacral incision to excise is frequently all that is required because they are so distally located and easily accessible. Teratomas often feature mature embryologic elements comprised of all three embryonic layers. Teeth, hair, sebaceous material, and bone can be found. They can be either cystic or solid or have elements of both and are often noted to be firmly adherent to the coccyx even when benign. As with ovarian teratomas, approximately 5–10% will undergo malignant degeneration (5).
All of these lesions are most often completely asymptomatic. They can (rarely) present with obstructed labor when large or alternatively be detected on a prenatal ultrasound or physical examination. Routine gynecologic examinations result in detection of some lesions, and others present with a vague, dull “pressure” sensation that calls attention to their presence (7). The most sensitive and specific test for diagnosis is magnetic resonance imaging (MRI) (8). The goals of imaging for any presacral mass
lesion are delineation of anatomy, characterization of the lesion, and assessment of adjacent tissue involvement. Preoperative biopsy of these discrete lesions is contraindicated to avoid infecting the lesion resulting in an infl ammatory reaction or infection and increasing the technical difficulty involved in excision. Elective excision is recommended, particularly with teratomas, where malignant degeneration, as mentioned, is a possibility. Otherwise, prognosis following successful resection of these lesions is 100%.
lesion are delineation of anatomy, characterization of the lesion, and assessment of adjacent tissue involvement. Preoperative biopsy of these discrete lesions is contraindicated to avoid infecting the lesion resulting in an infl ammatory reaction or infection and increasing the technical difficulty involved in excision. Elective excision is recommended, particularly with teratomas, where malignant degeneration, as mentioned, is a possibility. Otherwise, prognosis following successful resection of these lesions is 100%.
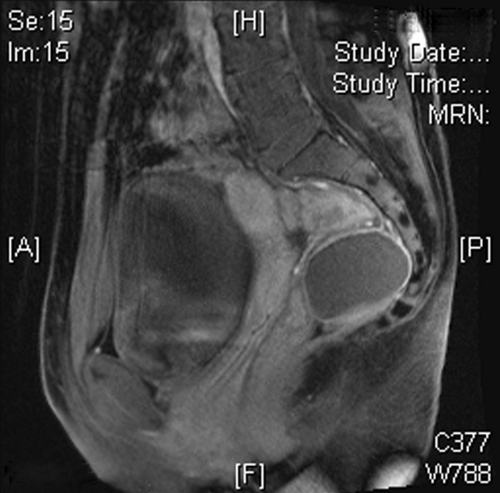 Figure 19.2 Sagittal view of dermoid cyst on magnetic resonance imaging. Presacral dermoid cyst in a young female. (Courtesy of Najjia N. Mahmoud, University of Pennsylvania.) |
Chordomas
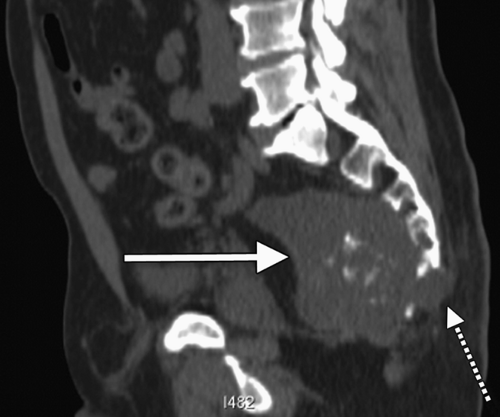
Chordomas are the most common malignant lesion in the presacral space and the second most common variety of retrorectal tumor (Fig. 19.3). It arises from the embryonic notochord and has a predilection for male gender 2:1 (3). Chordomas typically present in males aged 40–60. Patients present with symptoms implying neurologic involvement characterized by pain, urinary incontinence or retention, and impotency or erectile dysfunction. Lower extremity paralysis tends to be uncommon unless the tumor is located in the lumbar or thoracic vertebrae. Thirty to fifty percent of chordomas are located in the sacrococcygeal region (9). The radical surgery typically required to clear the tumor’s lateral margins frequently results in motor and sensory dysfunction. Chordomas are quite resistant to chemotherapy and are radiation-insensitive. Radical resection represents the only chance for cure. Survival rates after chordoma resection have been rising. In the 1970s, recurrence rates were reported in excess of 90% and survival was less than 20% following diagnosis (10). Advances in imaging, surgery, and reconstruction have been credited with improving survival four-fold. Even so, recurrence rates at 5 years are as high as 40–70%. Survival after local recurrence is rare (11).
Anterior Sacral Meningocele
Anterior sacral meningocele (ASM) is a rare developmental lesion with a slight female predominance precipitated by a congenital defect representing an area of sacral agenesis through which the dura herniates. ASM is associated with the Currarino syndrome or triad, an autosomal dominant condition that includes findings of presacral mass, sacral deformity, and anorectal malformation (12). It is most commonly, an isolated finding. The associated sac is filled with cerebrospinal fluid. Patients frequently complain of headaches, pressure, lower back pain, and urinary difficulty. Headache during defecation or obstructed defecation can result as well. Diagnosis is typically made by imaging studies such as computed tomography or MRI, but a classic, often mentioned finding on plain film is the “scimitar sign” associated with the herniation of the dural
sac (13) (Fig. 19.4). Biopsy of this lesion is absolutely contraindicated as it can cause cerebrospinal fluid leak and meningitis (13).
sac (13) (Fig. 19.4). Biopsy of this lesion is absolutely contraindicated as it can cause cerebrospinal fluid leak and meningitis (13).
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree