Gallstone disease is a frequent condition throughout the world and, cholesterol stones are the most frequent form in Western countries. The standard treatment of symptomatic gallstone subjects is laparoscopic cholecystectomy. The selection of patients amenable for nonsurgical, medical therapy is of key importance; a careful analysis should consider the natural history of the disease and the overall costs of therapy. Only patients with mild symptoms and small, uncalcified cholesterol gallstones in a functioning gallbladder with a patent cystic duct are considered for oral litholysis by hydrophilic ursodeoxycholic acid, in the hope of achieving cholesterol desaturation of bile and progressive stone dissolution. Recent studies have raised the possibility that cholesterol-lowering agents that inhibit hepatic cholesterol synthesis (statins) or intestinal cholesterol absorption (ezetimibe), or drugs acting on specific nuclear receptors involved in cholesterol and bile acid homeostasis, may offer, alone or in combination, additional medical therapeutic tools for treating cholesterol gallstones. Recent perspectives on medical treatment of cholesterol gallstone disease are discussed in this article.
Gallstone disease is one of the most frequent and costly digestive diseases in Western countries; its prevalence in adults ranges from 10% to 15%. Despite the frequency of the condition, many patients with gallstones remain undiagnosed, although symptoms and/or complications occur in approximately a third of patients. In the United States, medical expenses for the treatment of gallstones exceeded $6 billion in the year 2000. The prevalence of gallstones seems to be rising and approximately 1 million new cases are discovered each year. About 75% of the gallstones in the United States and Westernized countries, including Italy, are cholesterol gallstones. The remaining gallstones are pigment stones that contain less than 30% cholesterol by weight, which can be subclassified into 2 groups: black pigment stones (about 20% of all gallstones, found in the gallbladder and/or bile duct, containing mainly insoluble bilirubin pigment polymer mixed with calcium phosphate and carbonate, and cholesterol) and brown pigment stones (about 5% of all gallstones, found mainly in bile ducts, containing calcium bilirubinate, calcium palmitate, and stearate and cholesterol).
Cholesterol gallstones are associated with well-known risk factors, such as obesity, type 2 diabetes, dyslipidemia, and hyperinsulinemia, which are often components of the metabolic syndrome epidemic, which has a prevalence greater than 35% in the adult population and which continues to increase in Westernized countries. Epidemiologic surveys have observed that cholesterol cholelithiasis is prevalent in populations consuming a Western diet (ie, enriched in saturated fatty acids, cholesterol, and rapidly absorbed refined carbohydrates), rather than a more prudent diet (ie, enriched in monopolyunsaturated fats, fruit, vegetables, and low in refined carbohydrates) associated with physical activity. Thus, the prevalence of cholesterol gallstone disease is significantly higher in North and South American as well as European populations than in Asian and African populations. In China, the prevalence of cholesterol gallstones seems to increase with the Westernization of the traditional Chinese diet. Even in Japan, the adoption of Western-type dietary habits has resulted in a marked increase of the prevalence of cholesterol cholelithiasis over the past 40 years. As discussed later, high efficiency of intestinal cholesterol absorption and high dietary cholesterol seem to be key and independent risk factors for the formation of cholesterol gallstones. The complex pathogenesis of cholesterol gallstones depends on the concurrent existence of hepatic hypersecretion of cholesterol into bile, leading to bile supersaturation with cholesterol, accelerated nucleation/crystallization of cholesterol in gallbladder bile, impaired gallbladder motility leading to gallbladder stasis, and increased cholesterol availability from the small intestine, as well as LITH genes and genetic factors. A complex genetic basis plays a key role in determining individual predisposition to developing cholesterol gallstones in response to environmental factors. Some gallstone genes might also play a potential role, including some genes governing the nuclear bile acid receptors such as farnesoid X receptor (FXR). For example, FXR variants seem to affect gallbladder motor function and intestinal microflora in Mexicans, whereas functional variants in FXR might account for intrahepatic cholestasis of pregnancy in Whites, as well as being associated with other cholestatic and dyslipidemic disorders.
From a therapeutic point of view, although gallstone disease is frequent in the general population and the costs of therapeutic interventions are high, the natural history of the disease suggests restriction of the medical treatment of gallstones to a subgroup of symptomatic patients. The selection of patients eligible for medical or surgical therapy, therefore, is of key importance. The onset of biliary pain is the only suggestive marker of symptomatic gallstone disease, although it can be difficult to distinguish between symptomatic and asymptomatic patients in a random population of patients with gallstones. The diagnosis can be misleading if patients inadequately describe typical symptoms or suffer from highly atypical symptoms. Previously symptomatic patients who are symptom-free for 5 consecutive years should be included in the group of asymptomatic subjects again. After this time, the risk of pain attacks gradually decreases toward values similar to those of patients with asymptomatic gallstones. Classic drug therapy for cholesterol gallstones (ie, oral litholysis by the bile acid ursodeoxycholic acid [UDCA]) plays a limited role, but novel interesting therapeutic options might arise in the near future, related to the molecular mechanisms responsible for the formation of cholesterol gallstones. Such novel therapeutic approaches might involve subgroups of patients permanently or temporarily at risk for gallstone formation. Recent studies in animal models and humans have found that blocking the intestinal absorption of cholesterol with ezetimibe (EZT), the potent inhibitor of the Niemann-Pick C1-like 1 (NPC1L1) protein, may provide a novel powerful strategy for the medical treatment of cholesterol gallstones. Modification of the expression levels of specific nuclear receptors (NR) in the liver might also provide a clue for novel therapeutic approaches for cholesterol gallstones via manipulation of cholesterol and bile acid homeostasis. Current views and perspectives on medical treatment of cholesterol gallstone disease are discussed in this article.
Guidelines for management of gallstone disease
Gallbladder stones are frequently found in asymptomatic patients during routine abdominal ultrasonography, because in most cases (60%–80%) gallstones do not generate symptoms. Previous observations have shown that the average risk of developing symptomatic gallstones is 2.0% to 2.6% per year. By contrast, the presence of microstones and sludge in the gallbladder is a major risk factor for the development of biliary pain and complicated gallstone disease, and also plays a main role in the cause of acute otherwise idiopathic pancreatitis. Nevertheless, the yearly incidence of complications is low (0.3%), and the annual risk for gallbladder cancer is as low as 0.02%. Treatment of asymptomatic patients with gallstones, therefore, is not routinely recommended, as the overall risk of biliary colic, complications, and gallbladder cancer is low. Expectant management is considered the appropriate choice in most asymptomatic patients with gallstones (grade A). The decision is different in symptomatic patients with gallstones and should follow the algorithm depicted in Fig. 1 , in which surgery (namely laparoscopic cholecystectomy) represents the gold standard for treatment; oral litholysis with hydrophilic bile salts plays a limited role. Other nonsurgical (nonpharmacologic) therapies include direct contact dissolutions of gallstones using the potent cholesterol solvent methyl tert butyl ether (MTBE), and extracorporeal shock wave lithotripsy. Both options, however, have lost their popularity because of potential side effects (MTBE) and high postdissolution recurrence rate. Available medical treatments for gallstones are discussed in the following paragraphs and include the treatment of biliary colic (all types of stones), oral litholysis by hydrophilic bile acids and novel approaches with statins, EZT, and agonists/antagonists of NR (all for cholesterol gallstones).
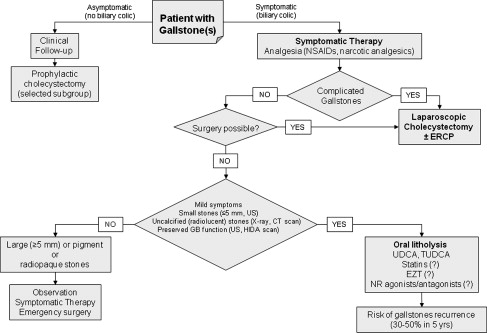
Medical treatments of gallstone disease
Treatment of the Biliary Colic
The presence of a gallstone of any type and size may put the patient at risk of biliary pain. As the intensity of pain is usually high (mean visual analog scale of 9 cm on a 0- to 10-cm scale), patients require immediate medical attention and analgesia. The pain is not exclusively postprandial, and is typically intermittent. The most frequent localization is the right upper quadrant of the abdomen and/or the epigastrium (representative dermatomes T8/9), and the duration is generally longer than 15 to 30 minutes. The pain radiates to the angle of the right scapula and/or shoulder in about 60% of cases. In less than 10% of cases the pain radiates to the retrosternal area. About two-thirds of patients experience an urge to walk, and often are nauseated or vomit. In biliary colic, the pain is visceral and is caused by the impaction of the stone in the cystic duct or the sphincter of Oddi. Distension of the gallbladder and/or biliary tract with activation of visceral sensory neurons may follow. The pain can last for several hours and be associated with nonspecific symptoms of indigestion. The pain can be relieved if the stone returns into the gallbladder lumen, passes through the sphincter into the duodenum, or migrates back to the common bile duct. The biliary pain is rapidly responsive to narcotic analgesics (meperidine ) or nonsteroidal anti-inflammatory drugs (NSAIDs) (such as intramuscular or intravenous ketorolac or ibuprofen by mouth), which could also reduce the risk of evolution toward acute cholecystitis. A second-line therapy includes the use of antispasmodic (anticholinergic) agents like hyoscine (scopolamine) which are known to be less effective than NSAIDs (grade A). The patient with biliary colic should remain fasting to avoid release of endogenous cholecystokinin and further gallbladder contraction. If a complicated biliary pain is suspected (association of leukocytosis, nausea, jaundice, vomiting, and fever), the patient should be quickly admitted to hospital and treated accordingly. Typical complications of gallstone disease are acute pancreatitis, acute cholecystitis, biliary obstruction and cholangitis, gallbladder perforation, abscess formation, and mucocele of the gallbladder, which may require additional medical therapy with antibiotics or invasive procedures with or without surgery. In mild and moderate acute cholecystitis, early laparoscopic cholecystectomy is recommended at between 2 and 4 days (grade A). The risk of biliary pain in asymptomatic carriers is estimated to be approximately 1% to 2% annually. Early studies that were not randomized or placebo-controlled found that UDCA, besides its litholytic effect (see later discussion), might also reduce the risk of biliary colic. In a nonrandomized study, Tomida and colleagues treated patients referred for symptomatic or asymptomatic gallstones with 600 mg UDCA per day and used those who refused as a control group. The incidence of biliary pain was apparently reduced by UDCA in asymptomatic patients, although a bias might include a misclassification of symptoms. However, in a large randomized, double-blind, placebo-controlled trial on the effects of UDCA in highly symptomatic patients with gallstones scheduled for cholecystectomy, UDCA did not exert a beneficial effect on biliary colic. The likelihood of remaining colic-free was comparable in patients with strong or weak baseline gallbladder contraction as determined by ultrasonography after a standard mixed meal.
Dissolution of Cholesterol Gallstones by Oral Bile Acids
About two-thirds of the gallstones in Western countries are composed mainly of cholesterol. However, dissolution therapy by oral administration of the hydrophilic bile acid UDCA, the 7β-epimer of chenodeoxycholate, is suitable only for a small subgroup (about 15%) of symptomatic patients. Similar results are reached with the taurine-conjugated UDCA (tauroursodeoxycholic acid [TUDCA]). Chances of dissolution are higher if gallstones are small (less than 0.5 cm in size), not calcified (radiolucent on radiograph, including a computed tomography [CT] scan), cholesterol-enriched (ie, more than 80%), and contained within a functioning gallbladder with a patent cystic duct. Complete dissolution of gallstones by bile acids was first documented by Rewbridge in 1937, although initial reports were published in 1873 and 1876. The bile acid chenodeoxycholic acid (CDCA) was first used in the 1970s but was associated with a dose-dependent increase in serum aminotransferases, serum low-density lipoprotein cholesterol levels, and diarrhea. In 1975 Makino and colleagues identified UDCA as a more hydrophilic bile acid that could replace CDCA without side effects. Dissolution of cholesterol gallstones by UDCA following fragmentation by extracorporeal shock wave lithotripsy was introduced first by Sauerbruch and colleagues in Munich in 1986. The bile acid UDCA is currently used for oral dissolution at a dosage of 10 to 14 mg/kg body weight per day. Bedtime administration is suggested because it maintains hepatic bile acid secretion rate overnight, thus reducing secretion of supersaturated bile and increasing the dissolution rate (grade A). Oral UDCA (at least 10 mg/kg/d) results in an increased proportion of biliary UDCA in bile (from less than 8%–10% of biliary bile acid pool to about 40%). Increasing biliary UDCA, in turn, results in a decreased hepatic secretion of biliary cholesterol and the formation of unsaturated gallbladder bile (ie, containing less cholesterol in solution) with a cholesterol saturation index of less than 1 ( Fig. 2 ). This step represents a key factor in initiating the process of dissolution of cholesterol crystals and gallstones. During UDCA treatment, cholesterol crystallization can be prevented because more cholesterol can be transported within vesicles that contain mainly phospholipids and cholesterol and little bile acid. Also, oral therapy with UDCA is associated with the reduction of intestinal absorption of cholesterol, as well as with a better contractility of the stimulated gallbladder smooth muscle, as shown by in vitro studies in animals and patients with gallstones. By decreasing cholesterol saturation of bile, UDCA might counteract the impaired contractility caused by incorporation of excessive luminal cholesterol into the plasmalemma of gallbladder smooth muscles. UDCA might also counteract the detrimental effects of the hydrophobic bile acid deoxycholate on the gallbladder smooth muscle contractility, and have an effect on local oxidative stress and risk of acute cholecystitis. Excess biliary cholesterol might provide the basis for stimulation of inflammatory cells in the gallbladder, because cholesterol monohydrate crystals induce expression of T-cell–dependent proinflammatory cytokines in a murine model of cholesterol cholelithogenesis.
Patients suitable for medical dissolution of cholesterol gallstones need to be carefully selected. Well-selected patients are those who have the higher chance for successful oral litholysis alone or after extracorporeal shock wave lithotripsy for stone fragmentation. Ultrasonography of the right upper quadrant is still the best and more convenient diagnostic tool for detecting gallstones, as well as for assessing gallstone size and burden. Functional ultrasonography, (ie, the study of time-dependent changes of fasting and postprandial gallbladder volumes following a standard test meal), although not routinely used, provides additional information about gallbladder size, emptying, and bile duct patency. Abdominal plain radiography (not routinely used) and the CT scan detect only calcified stones (as radiopaque bodies) in the right upper quadrant. Such stones are unfit for dissolution because they are either calcified cholesterol stones or stones made of pigment calcium bilirubinate. Oral cholecystography might disclose the presence of floating (cholesterol) stones in the gallbladder and a preserved cystic duct patency. The expected dissolution rate following UDCA at the standard dosage is estimated to be about a 1-mm decrement in stone diameter per month. In patients with a gallstone diameter less than 5 mm, the complete disappearance of stones assessed by ultrasonography is expected to be reached in about 90% of cases by 6 months of UDCA administration. A series of conditions might impede the dissolution of cholesterol gallstones by UDCA or TUDCA. In patients with larger and/or multiple stones the dissolution rate approaches 40% to 50% after 1 year of the treatment, whereas the appearance of a surface calcification on cholesterol gallstones during oral dissolution therapy with UDCA, CDCA, or TUDCA has been reported in about 10% to 12% of patients. This event would impede any further dissolution of the calcified stone. Another issue is the possibility that gallstones will recur sometime after dissolution with bile acids, and this is a major limitation of oral dissolution therapy. Overall, recurrence might be as high as 10% per year (ie, about 30%–50% of cases) 5 years after bile acid therapy or lithotripsy. Recurrence rate is higher particularly in patients with multiple gallstones. Whereas recurrent gallstones respond well to retreatment, such a high recurrence rate may be dependent on persistent pathogenetic conditions. Oral dissolution therapy for cholesterol gallstones with bile acids might still represent the option in patients who are at minimal risk of gallstone recurrence or have transient risk factors including rapid weight loss (ie, obese patients following bariatric surgery), pregnancy, and convalescence from abdominal surgery. Major limitations for oral litholysis with bile acids, by contrast, are the small number of suitable patients and the high rate of gallstone recurrence.
Medical treatments of gallstone disease
Treatment of the Biliary Colic
The presence of a gallstone of any type and size may put the patient at risk of biliary pain. As the intensity of pain is usually high (mean visual analog scale of 9 cm on a 0- to 10-cm scale), patients require immediate medical attention and analgesia. The pain is not exclusively postprandial, and is typically intermittent. The most frequent localization is the right upper quadrant of the abdomen and/or the epigastrium (representative dermatomes T8/9), and the duration is generally longer than 15 to 30 minutes. The pain radiates to the angle of the right scapula and/or shoulder in about 60% of cases. In less than 10% of cases the pain radiates to the retrosternal area. About two-thirds of patients experience an urge to walk, and often are nauseated or vomit. In biliary colic, the pain is visceral and is caused by the impaction of the stone in the cystic duct or the sphincter of Oddi. Distension of the gallbladder and/or biliary tract with activation of visceral sensory neurons may follow. The pain can last for several hours and be associated with nonspecific symptoms of indigestion. The pain can be relieved if the stone returns into the gallbladder lumen, passes through the sphincter into the duodenum, or migrates back to the common bile duct. The biliary pain is rapidly responsive to narcotic analgesics (meperidine ) or nonsteroidal anti-inflammatory drugs (NSAIDs) (such as intramuscular or intravenous ketorolac or ibuprofen by mouth), which could also reduce the risk of evolution toward acute cholecystitis. A second-line therapy includes the use of antispasmodic (anticholinergic) agents like hyoscine (scopolamine) which are known to be less effective than NSAIDs (grade A). The patient with biliary colic should remain fasting to avoid release of endogenous cholecystokinin and further gallbladder contraction. If a complicated biliary pain is suspected (association of leukocytosis, nausea, jaundice, vomiting, and fever), the patient should be quickly admitted to hospital and treated accordingly. Typical complications of gallstone disease are acute pancreatitis, acute cholecystitis, biliary obstruction and cholangitis, gallbladder perforation, abscess formation, and mucocele of the gallbladder, which may require additional medical therapy with antibiotics or invasive procedures with or without surgery. In mild and moderate acute cholecystitis, early laparoscopic cholecystectomy is recommended at between 2 and 4 days (grade A). The risk of biliary pain in asymptomatic carriers is estimated to be approximately 1% to 2% annually. Early studies that were not randomized or placebo-controlled found that UDCA, besides its litholytic effect (see later discussion), might also reduce the risk of biliary colic. In a nonrandomized study, Tomida and colleagues treated patients referred for symptomatic or asymptomatic gallstones with 600 mg UDCA per day and used those who refused as a control group. The incidence of biliary pain was apparently reduced by UDCA in asymptomatic patients, although a bias might include a misclassification of symptoms. However, in a large randomized, double-blind, placebo-controlled trial on the effects of UDCA in highly symptomatic patients with gallstones scheduled for cholecystectomy, UDCA did not exert a beneficial effect on biliary colic. The likelihood of remaining colic-free was comparable in patients with strong or weak baseline gallbladder contraction as determined by ultrasonography after a standard mixed meal.
Dissolution of Cholesterol Gallstones by Oral Bile Acids
About two-thirds of the gallstones in Western countries are composed mainly of cholesterol. However, dissolution therapy by oral administration of the hydrophilic bile acid UDCA, the 7β-epimer of chenodeoxycholate, is suitable only for a small subgroup (about 15%) of symptomatic patients. Similar results are reached with the taurine-conjugated UDCA (tauroursodeoxycholic acid [TUDCA]). Chances of dissolution are higher if gallstones are small (less than 0.5 cm in size), not calcified (radiolucent on radiograph, including a computed tomography [CT] scan), cholesterol-enriched (ie, more than 80%), and contained within a functioning gallbladder with a patent cystic duct. Complete dissolution of gallstones by bile acids was first documented by Rewbridge in 1937, although initial reports were published in 1873 and 1876. The bile acid chenodeoxycholic acid (CDCA) was first used in the 1970s but was associated with a dose-dependent increase in serum aminotransferases, serum low-density lipoprotein cholesterol levels, and diarrhea. In 1975 Makino and colleagues identified UDCA as a more hydrophilic bile acid that could replace CDCA without side effects. Dissolution of cholesterol gallstones by UDCA following fragmentation by extracorporeal shock wave lithotripsy was introduced first by Sauerbruch and colleagues in Munich in 1986. The bile acid UDCA is currently used for oral dissolution at a dosage of 10 to 14 mg/kg body weight per day. Bedtime administration is suggested because it maintains hepatic bile acid secretion rate overnight, thus reducing secretion of supersaturated bile and increasing the dissolution rate (grade A). Oral UDCA (at least 10 mg/kg/d) results in an increased proportion of biliary UDCA in bile (from less than 8%–10% of biliary bile acid pool to about 40%). Increasing biliary UDCA, in turn, results in a decreased hepatic secretion of biliary cholesterol and the formation of unsaturated gallbladder bile (ie, containing less cholesterol in solution) with a cholesterol saturation index of less than 1 ( Fig. 2 ). This step represents a key factor in initiating the process of dissolution of cholesterol crystals and gallstones. During UDCA treatment, cholesterol crystallization can be prevented because more cholesterol can be transported within vesicles that contain mainly phospholipids and cholesterol and little bile acid. Also, oral therapy with UDCA is associated with the reduction of intestinal absorption of cholesterol, as well as with a better contractility of the stimulated gallbladder smooth muscle, as shown by in vitro studies in animals and patients with gallstones. By decreasing cholesterol saturation of bile, UDCA might counteract the impaired contractility caused by incorporation of excessive luminal cholesterol into the plasmalemma of gallbladder smooth muscles. UDCA might also counteract the detrimental effects of the hydrophobic bile acid deoxycholate on the gallbladder smooth muscle contractility, and have an effect on local oxidative stress and risk of acute cholecystitis. Excess biliary cholesterol might provide the basis for stimulation of inflammatory cells in the gallbladder, because cholesterol monohydrate crystals induce expression of T-cell–dependent proinflammatory cytokines in a murine model of cholesterol cholelithogenesis.
Patients suitable for medical dissolution of cholesterol gallstones need to be carefully selected. Well-selected patients are those who have the higher chance for successful oral litholysis alone or after extracorporeal shock wave lithotripsy for stone fragmentation. Ultrasonography of the right upper quadrant is still the best and more convenient diagnostic tool for detecting gallstones, as well as for assessing gallstone size and burden. Functional ultrasonography, (ie, the study of time-dependent changes of fasting and postprandial gallbladder volumes following a standard test meal), although not routinely used, provides additional information about gallbladder size, emptying, and bile duct patency. Abdominal plain radiography (not routinely used) and the CT scan detect only calcified stones (as radiopaque bodies) in the right upper quadrant. Such stones are unfit for dissolution because they are either calcified cholesterol stones or stones made of pigment calcium bilirubinate. Oral cholecystography might disclose the presence of floating (cholesterol) stones in the gallbladder and a preserved cystic duct patency. The expected dissolution rate following UDCA at the standard dosage is estimated to be about a 1-mm decrement in stone diameter per month. In patients with a gallstone diameter less than 5 mm, the complete disappearance of stones assessed by ultrasonography is expected to be reached in about 90% of cases by 6 months of UDCA administration. A series of conditions might impede the dissolution of cholesterol gallstones by UDCA or TUDCA. In patients with larger and/or multiple stones the dissolution rate approaches 40% to 50% after 1 year of the treatment, whereas the appearance of a surface calcification on cholesterol gallstones during oral dissolution therapy with UDCA, CDCA, or TUDCA has been reported in about 10% to 12% of patients. This event would impede any further dissolution of the calcified stone. Another issue is the possibility that gallstones will recur sometime after dissolution with bile acids, and this is a major limitation of oral dissolution therapy. Overall, recurrence might be as high as 10% per year (ie, about 30%–50% of cases) 5 years after bile acid therapy or lithotripsy. Recurrence rate is higher particularly in patients with multiple gallstones. Whereas recurrent gallstones respond well to retreatment, such a high recurrence rate may be dependent on persistent pathogenetic conditions. Oral dissolution therapy for cholesterol gallstones with bile acids might still represent the option in patients who are at minimal risk of gallstone recurrence or have transient risk factors including rapid weight loss (ie, obese patients following bariatric surgery), pregnancy, and convalescence from abdominal surgery. Major limitations for oral litholysis with bile acids, by contrast, are the small number of suitable patients and the high rate of gallstone recurrence.
Novel medical treatments
The presence of a lithogenic bile is primarily a result of a sustained hypersecretion of biliary cholesterol, which has 2 key components: hepatic and intestinal. In principle, drugs influencing hepatic synthesis and/or secretion of cholesterol (ie, statins) and/or intestinal absorption of cholesterol (ie, EZT) are potentially able to influence the formation of cholesterol gallstones and to promote dissolution of gallstones.
Inhibition of Hepatic Cholesterol Synthesis by Statins
Statins are competitive inhibitors of 3-hydroxy-3-methylglutaryl coenzyme A (HMG CoA) reductase, the rate-limiting step in cholesterol biosynthesis. They occupy a portion of the binding site of HMG CoA, blocking access of this substrate to the active site on the enzyme. Currently available statins in the United States include lovastatin, pravastatin, simvastatin, fluvastatin, atorvastatin, and rosuvastatin. Statins seem also to reduce cholesterol secretion and concentration in bile independently of their ability to block hepatic cholesterol synthesis. Such combined effects of statins on cholesterol homeostasis in the liver and bile might be able to lower the risk of cholesterol gallstones. Beneficial effects of statins in preventing gallstone formation have been reported in animal studies. In humans the effect of statins on gallstone disease has been controversial; reduced gallstone formation, decreased cholesterol concentration in bile, and gallstone dissolution following therapy with statins have been reported by some, but not all, studies. Another 2 small studies have also been conflicting, with either no association between statin use and the risk of gallstones or with an effect of statin on gallstones, although the statistical power was small. More recently, 2 studies have reassessed the problem of statin use and risk of gallstone disease, and opened new perspectives. In a cohort of US women self-reporting long-term use statins, the risk of cholecystectomy was found to decrease slightly. In a case-control analysis using the United Kingdom–based General Practice Research Database and evaluating incident patients between 1994 and 2004, long-term use of statins (1–1.5 years) was associated with a decreased risk of gallstones followed by cholecystectomy, compared with patients without statin use. Whether statin use will be part of the medical therapeutic armamentarium in a subgroup of patients with gallstone disease or to prevent gallstone disease in selected patients at risk needs to be investigated further by appropriate clinical studies.
Inhibition of Intestinal Cholesterol Absorption by EZT
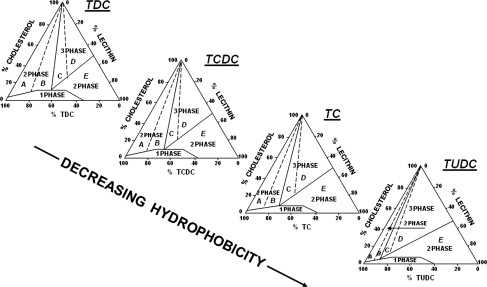
The importance of intestinal factors in the pathogenesis of cholesterol gallstones has recently been investigated by several research groups. Animal studies have shown that when no dietary cholesterol is available, all biliary cholesterol is mainly derived from hepatic de novo synthesis with a limited contribution (less than 15%) to biliary cholesterol secretion. Rather, the small intestine is the site that solely provides the absorption of dietary cholesterol, as well as reabsorption of biliary cholesterol. The importance of intestinal absorption of cholesterol for gallstone pathogenesis is supported by the positive correlation between the efficiency of intestinal cholesterol absorption and the prevalence of cholesterol gallstone formation in several strains of inbred mice. The protein NPC1L1 is highly expressed in the small intestine and localized along the brush border of the enterocytes in humans and mice. There is also a significant amount of NPC1L1 in the human liver but not in the mouse liver. Cholesterol is the most effective substrate of NPC1L1, which governs intestinal absorption of cholesterol by recycling between endocytic recycling compartment and plasma membrane ( Fig. 3 ). Thus, inhibition of cholesterol absorption in the intestine or hepatic uptake of chylomicron remnants has become an attractive possibility to decrease biliary cholesterol secretion and saturation. Similar to humans, the abundance of NPC1L1 in the small intestine far exceeded that in other regions of the gastrointestinal tract, liver, and gallbladder in the Golden Syrian hamster. EZT-induced reduction in intestinal cholesterol absorption is coupled with a decrease in the absolute and relative cholesterol levels in bile in hamsters fed a high-cholesterol and high-fat diet. These results are consistent with the recent finding that EZT treatment significantly reduced biliary cholesterol saturation in patients with gallstones. EZT belongs to the new class of 2-azetidinones approved as a novel hypocholesterolemic drug with a potent inhibitory effect on intestinal cholesterol absorption by specifically suppressing the NPC1L1. EZT might therefore play a primary role in the medical treatment or prevention of cholesterol gallstones, as suggested by studies from the authors’ group ( Fig. 4 ). In mice, EZT reduces cholesterol and partly phospholipid but not bile salt content in gallbladder bile; all crystallization pathways and phase boundaries in the bile phase diagram remain similar, with or without EZT. If EZT is increased, the relative lipid compositions of pooled gallbladder bile samples are progressively shifted down and to the left of the phase diagram, and enter the 1-phase (protective) micellar zone (which contains an abundance of unsaturated micelles but never solid cholesterol crystals or liquid crystals). Thus, the micellar cholesterol solubility is increased in gallbladder bile with more cholesterol molecules transferred from the cholesterol monohydrate surface into unsaturated micelles. In this environment, gallstones can dissolve. EZT also protected gallbladder motility function by desaturating bile. The physical-chemical mechanisms underlying the beneficial effects of EZT on supersaturated bile, cholesterol crystals, and cholesterol stones differ from those of hydrophilic bile acids such as UDCA, TUDCA and β-muricholic acid. These hydrophilic bile acids enhance dissolution of cholesterol gallstones by promoting the formation of a vesicle-enriched liquid crystalline mesophase. Translational studies have shown that EZT (20 mg/d by mouth for 1 month) significantly reduced cholesterol concentration and cholesterol saturation index and retarded cholesterol crystallization in patients with gallstones.






