Surgical Issues in the Transplant Recipient
David B. Leeser
Stephen T. Bartlett
Division of Transplantation, Department of Surgery, University of Maryland School of Medicine, Baltimore, Maryland 21201
PRETRANSPLANT CARE AND EVALUATION BY THE SURGEON
The treatment of the kidney transplant recipient by the transplant surgeon begins with the pretransplant evaluation. At this time, the surgeon along with nephrologists, social workers, dietitians, transplant coordinators, and cardiologists perform a thorough work-up to determine a patient’s suitability for cadaveric or living donor renal transplantation. The evaluation process is used to select appropriate patients for placement on the waiting list or further preparation toward living donor transplantation and is covered in detail elsewhere in this text. Issues of specific importance to the surgeon include the patient’s previous surgical history, vascular examination, overall risk for anesthetic complications, and the presence of any pathologic process that might require pretransplant surgical treatment (i.e., inguinal hernia, biliary disease, peripheral vascular disease, symptomatic polycystic kidney disease).
SURGICAL DISEASE IN THE PRETRANSPLANT PERIOD
Patients found to have any type of abdominal wall hernia during evaluation for organ transplant should be referred for evaluation and repair by a qualified general surgeon. The list of possible complications of untreated hernia can be devastating in the healthy patient, and these risks are only magnified in the immunosuppressed recipient. Second, steroids and immunosuppressants are known to compromise wound healing (1). Therefore, all hernias should be repaired prior to transplantation.
The treatment of biliary disease in the patient with renal failure being evaluated for possible kidney transplantation is an area of controversy. General agreement exists that symptomatic cholelithiasis should be treated by laparoscopic cholecystectomy. Patient history should include questions that elicit a history of right upper quadrant pain, fatty or acholic stools, intolerance of fatty foods, jaundice, or pancreatitis. The presence of any of these in the history should prompt evaluation with a right upper quadrant ultrasound. The difficulties lie in the treatment of asymptomatic cholelithiasis in the pretransplant period. Many institutions perform screening abdominal ultrasonography as part of the pretransplant evaluation. Gallstones have been estimated to be present in anywhere from 7% to 40% of transplant candidates (2, 3, 4). The potential for serious complications from symptomatic gallstone disease in the transplant recipient has been well described in the literature and includes jaundice, pancreatitis, loss of the allograft, and death (5, 6, 7, 8, 9). The potential for serious adverse events and the relatively low morbidity and mortality has led to the suggestion that prophylactic laparoscopic cholecystectomy should be performed in all candidates for solid organ transplant prior to transplantation (5,10). Others have concluded that the morbidity and mortality of asymptomatic gallstones are overstated and that the percentage of patients prevented from developing complications does not justify the risk of prophylactic surgery, since up to 82% will never develop symptoms (2). No definitive prospective trials have conclusively settled this issue,
and a meta-analysis of the available literature was unable to provide any firm conclusions or recommendations (11). Therefore, the treatment of pretransplant cholelithiasis is center specific. At our center, we do not currently perform prophylactic laparoscopic cholecystectomy prior to renal transplantation. During the era of using routine muromonab-CD3 (OKT3) induction, one frequently observed episodes of posttransplant cholecystitis. In an era of induction agents that do not lead to cytokine release (i.e., interleukin-2 receptor antibody), we have dropped prophylactic cholecystectomy.
and a meta-analysis of the available literature was unable to provide any firm conclusions or recommendations (11). Therefore, the treatment of pretransplant cholelithiasis is center specific. At our center, we do not currently perform prophylactic laparoscopic cholecystectomy prior to renal transplantation. During the era of using routine muromonab-CD3 (OKT3) induction, one frequently observed episodes of posttransplant cholecystitis. In an era of induction agents that do not lead to cytokine release (i.e., interleukin-2 receptor antibody), we have dropped prophylactic cholecystectomy.
The treatment of peripheral vascular disease (PVD) in the patient population with chronic renal failure is another area of controversy. The pretransplant history should include questions regarding nonhealing ulcers on the lower extremities, claudication, rest pain, and any current or past tobacco use. Physical examination should include upper and lower extremity pulse examination. A positive history should prompt noninvasive vascular studies, which include anklebrachial indices with Doppler-derived segmental pressures of both lower extremities. If femoral pulses are diminished, then the patient should also have an aortogram with runoff or a magnetic resonance angiogram (MRA) of the abdominal aorta. If peripheral vascular disease is confirmed, the treatment plan is an area of debate. The survival of the patients with end-stage renal disease (ESRD) following lower extremity bypass for limb salvage has been reported to be 69%, 18%, and 5% at 1, 3, and 5 years, respectively (12). The poor outcome may be contributed to by the increased mortality from ESRD, but has led some to suggest consideration of early amputation in this patient population (13,14). Others have acknowledged the higher mortality rates in patients with ESRD and peripheral vascular disease, but continue to advocate attempts at limb salvage in this patient group. Limb salvage rates are reported to be between 71% and 80% at 2 years (12,15). A review of patients with renal transplants and peripheral vascular disease at our institution found patient survival at 1 and 5 years following infrainguinal vascular reconstruction to be 96% and 72%, respectively (unpublished data). Since kidney transplantation is known to improve survival substantially over hemodialysis, we recommend aggressive treatment of PVD in renal transplant candidates.
Candidates for renal transplantation should also be evaluated for aortic aneurysm. Important risk factors for aneurysm formation are the presence of hypertension and a history of smoking in the patient. Many candidates for renal transplantation have these risk factors. The abdomen should be examined for a palpable mass, and if one is appreciated, an abdominal ultrasound should be performed. In patients found to have infrarenal aortic aneurysmal disease, the repair can be done as part of a simultaneous aneurysm repair and living donor transplant or as a staged procedure while a patient waits for a cadaver kidney. We have performed simultaneous aortic bypass and living donor transplantation in nine patients and aortic repair followed by cadaveric renal transplant in six patients. Five-year survival was 87% in this group of patients. Two grafts were lost secondary to polyoma virus and noncompliance. Repair of aortic aneurysm is also possible in patients that have previously received a renal allograft. In these cases, the procedure must be planned to minimize the ischemic period experienced by the kidney that receives blood from the iliac artery below the aneurysm repair site. If the iliac arteries are involved with the aneurysm, the kidney may need to be retransplanted during the aneurysm repair. These procedures should involve the combined efforts of a vascular and transplant surgeon (16,17).
Cerebral vascular pathology that can also be found in the population with ESRD is cerebrovascular occlusive disease. Patients with ESRD have a relative risk of stroke that is 6.1 times greater than the general population (18). Fifty-four percent to 61% of patients with renal failure have measurable plague in the carotid artery (19). Patients should be queried about any history of stroke or symptoms of transient ischemic attack. The patient should be examined for carotid bruits or if a significant history of peripheral vascular or cardiovascular occlusive disease exists, a carotid duplex ultrasound should be performed to rule out carotid artery stenosis. If significant stenosis is found, the patient should be referred to a vascular surgeon for evaluation for carotid endarterectomy. For symptomatic patients, a stenosis of 50% or greater should be considered for treatment by endarterectomy (20). In patients without symptoms, a stenosis of 60% or greater should be considered for treatment by endarterectomy as long as the treating surgeon has a stroke rate of less than 3%. These recommendations are consistent with the Asymptomatic Carotid Artery Stenosis Trial and the North American Symptomatic Carotid Endarterectomy Trial (21).
Patients with renal failure secondary to polycystic kidney disease should be asked about symptoms related to their disease process, which may warrant native nephrectomy. In some cases, patients complain of increased abdominal girth, early satiety, respiratory compromise, hematuria, recurrent urinary tract infections, and abdominal pain which can be a result of the native kidneys. The patient should be examined for palpable masses on either side of the midline and to ascertain whether there will be adequate room for the allograft. In cases where the kidneys have enlarged to such an extent as to cause symptoms or are causing significant hematuria or infection, the patient should be evaluated with a computed tomography (CT) scan and native bilateral nephrectomy considered. The native nephrectomy can be planned as a staged procedure, or can be done at the time of kidney transplantation, or performed after transplantation when the physiologic condition of the patient has improved. Treatment of patients with polycystic kidney disease requiring native nephrectomy should involve a collaborative effort between a urologist and transplant surgeon (22).
Finally, patients with severe hypertension requiring three drugs or more; with diastolic pressures greater than 115; with onset before age 30 or after age 55; or with significant
cardiovascular or PVD should prompt consideration of renovascular hypertension. Renovascular hypertension occurs when uni- or bilateral stenosis or occlusion of the renal arteries results in a diminution of the pressure experience by the juxtaglomerular complex. The resultant secretion of renin activates angiotensinogen to angiotensin I. Angiotensin I is then converted to angiotensin II which causes direct vasoconstriction and also stimulates the release of aldosterone which causes sodium and volume retention. Renal artery stenosis is caused most commonly by atherosclerotic disease in 75% to 95% of cases and fibromuscular dysplasia in 5% to 20%. The diagnosis of renovascular hypertension is confirmed by captopril renography (23, 24, 25). Captopril renography involves baseline radionuclide scintigraphy followed by dosing with captopril and repeat scintigraphy after 1 hour. A positive scan is indicated if asymmetric excretion of tracer is noted before or after captopril, if kidney size is small or asymmetric, or if tracer excretion is diminished after captopril. The concurrence of renal artery stenosis is confirmed by conventional arteriography or magnetic resonance arteriography (24). In patients with established renal failure, the diagnosis is confirmed by angiography alone, and the renal scan can be omitted. In the presence of renal failure and confirmed renovascular hypertension, strong consideration should be given to nephrectomy prior to or concurrently with renal transplantation. In some cases, renal function can be restored by performing renal artery bypass or balloon angioplasty to the affected kidney. The preservation of native renal function should be attempted whenever possible. Occasionally, patients with ESRD can come off hemodialysis after correction of renal artery stenosis (26).
cardiovascular or PVD should prompt consideration of renovascular hypertension. Renovascular hypertension occurs when uni- or bilateral stenosis or occlusion of the renal arteries results in a diminution of the pressure experience by the juxtaglomerular complex. The resultant secretion of renin activates angiotensinogen to angiotensin I. Angiotensin I is then converted to angiotensin II which causes direct vasoconstriction and also stimulates the release of aldosterone which causes sodium and volume retention. Renal artery stenosis is caused most commonly by atherosclerotic disease in 75% to 95% of cases and fibromuscular dysplasia in 5% to 20%. The diagnosis of renovascular hypertension is confirmed by captopril renography (23, 24, 25). Captopril renography involves baseline radionuclide scintigraphy followed by dosing with captopril and repeat scintigraphy after 1 hour. A positive scan is indicated if asymmetric excretion of tracer is noted before or after captopril, if kidney size is small or asymmetric, or if tracer excretion is diminished after captopril. The concurrence of renal artery stenosis is confirmed by conventional arteriography or magnetic resonance arteriography (24). In patients with established renal failure, the diagnosis is confirmed by angiography alone, and the renal scan can be omitted. In the presence of renal failure and confirmed renovascular hypertension, strong consideration should be given to nephrectomy prior to or concurrently with renal transplantation. In some cases, renal function can be restored by performing renal artery bypass or balloon angioplasty to the affected kidney. The preservation of native renal function should be attempted whenever possible. Occasionally, patients with ESRD can come off hemodialysis after correction of renal artery stenosis (26).
PERITRANSPLANT CARE
Aside from brief visits to update a patient’s work-up while on the waiting list, the surgeon’s interaction with the transplant candidate is minimal until a call from an organ procurement organization (OPO) identifies the candidate as a possible recipient for a kidney. When a kidney becomes available, the surgeon and a transplant coordinator contact potential recipients and their nephrologists to determine their immediate medical readiness for transplant. The patient should also be asked about their desire to proceed with the organ being offered if the donor fits expanded criteria. The patient is then brought to the hospital while crossmatching is performed to ensure that no barrier exists to a successful transplant.
Crossmatching is performed in order to prevent the occurrence of hyperacute antibody-mediated rejection and accelerated forms of acute rejection in the transplanted organs due to the presence of preformed anti-human leukocyte antigen (HLA) antibodies in the recipient to donor lymphocytes. Three types of crossmatching procedures currently exist. The first is the direct complement-dependent lymphocytotoxicity (CDC) test in which recipient sera is placed with donor lymphocytes and the cells are monitored for the occurrence of complement-mediated cell lysis. The second crossmatch procedure is the anti-human globulin (AHG)-enhanced CDC in which rabbit complement is used to increase the sensitivity of the test. Finally, the most sensitive test is the flow cytometry crossmatch in which recipient sera is placed with donor lymphocytes and then fluorescent antibodies are used to detect recipient antibodies adherent to the donor lymphocytes. Most centers use the AHG test for patients without a history of prior sensitization from previous transplants, blood transfusions, or pregnancies. However, if patients are found to have anti-HLA antibodies on a panel of reactive antibodies (PRA), which measures the presence of antibodies to common HLA types, then the type of crossmatch used is center dependent. The specificity of anti-HLA antibodies can also be identified so that donors with these HLA types can be excluded from consideration. Our practice is to perform a flow cytometric crossmatch on all patients with a history of prior transplantation or a PRA greater than 40%, while other centers feel that the flow crossmatch is too time-consuming and too sensitive.
Once the patient arrives at the hospital, a thorough history is taken and a physical performed. The history should include attention to any interim cardiac events, recent illnesses, active PVD, and the date and time of the patient’s last hemodialysis treatment. These questions should elicit any recent changes in the recipient’s medical condition that would preclude transplantation due to an acute illness or recent event that would make risk of anesthesia too high. The patient should also be queried for the amount of urine produced on a daily basis to aid in postoperative management. Physical exam should be comprehensive with particular attention to signs of congestive heart failure, and include a lower extremity vascular examination and the presence and type of dialysis access. Laboratory tests should include a complete blood count (CBC); basic metabolic panel; calcium, magnesium, and phosphorous levels; coagulation tests; and type and crossmatch. If there is any indication of fluid overload or a potassium level greater than 5.3, nephrology should be contacted immediately and hemodialysis should be considered prior to the transplant procedure (27,28).
Once the above is done, our practice is to complete and review a pretransplant checklist (Fig. 8.1). The patient’s name, medical record number, and social security number are checked and recorded from the hospital admission data. Blood type is checked on the admission type and screen. The patient’s serologies are then recorded from original laboratory reports. The donor United Network for Organ Sharing (UNOS) number is recorded from the data received with the organ and the match of the patient’s name and social security number with the organ is confirmed with the OPO. The serologies and blood type of the donor are recorded from primary source documents of the donor hospital to ensure a proper match between donor and recipient. The crossmatch data is reviewed and recorded. Once these aspects of the checklist are recorded, the information is reviewed
with the patient, while maintaining appropriate donor confidentiality; as part of the informed consent process and consent for transplantation, consent for expanded criteria (hepatitis B- or hepatitis C-positive donors) is obtained. The checklist process ensures the transplantation of appropriate organs into appropriate patients.
with the patient, while maintaining appropriate donor confidentiality; as part of the informed consent process and consent for transplantation, consent for expanded criteria (hepatitis B- or hepatitis C-positive donors) is obtained. The checklist process ensures the transplantation of appropriate organs into appropriate patients.
Once the evaluation by the transplant surgeon is complete, the patient can be given preoperative immunosuppressants, which can include a calcineurin inhibitor and mycophenolate mofetil. Orders are written for the patient to receive prophylactic perioperative antibiotics, steroids and an immunosuppressive induction agent like basiliximab or antithymocyte immunoglobulin intraoperatively.
OPERATIVE PROCEDURES
Prior to bringing the patient to the operating room, the organ is removed from storage on iced University of Wisconsin solution and placed in a basin with slush for back table preparation. The perinephric fat is removed and the hilar fat is dissected away from the artery and vein to free an adequate length to perform vascular anastomoses. Arterial and venous branches to the adrenal gland are ligated and divided and the gland removed. The gonadal vein is identified and ligated. The gonadal vein can be saved should an extension graft be needed for arterial reconstruction. Excessive periureteral tissue is also removed, taking care to preserve the immediate periureteral tissue, which carries the blood supply. The kidney is also inspected for any pathology not seen by the procuring surgeon that would preclude transplantation prior to the patient’s arrival in the operating room.
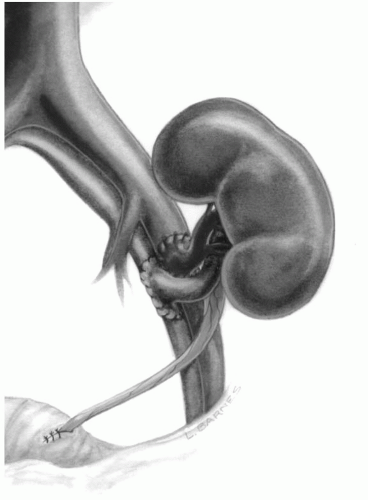
The patient is brought to the operating room and general anesthesia is induced. A central venous or Swan-Ganz catheter is placed. An arterial line is optional. A Foley catheter is placed and approximately 200 cc of antibiotic solution is placed in the bladder. Compression stockings and sequential compression devices are placed on the patient’s lower extremities. The abdomen is prepped and draped. Perioperative antibiotics, steroids, and, if desired, thymocyte-depleting induction agents are given to the patient. Routinely, a right lower quadrant hockey stick incision is made. The right side is preferred since the iliac vessels tend to be more superficial allowing the construction of the vascular anastomoses to be carried out more easily. Using cautery, the incision is carried down through the aponeurosis of the external oblique. The abdominal oblique musculature is then divided to expose the peritoneum. The peritoneum is swept medially, so that the abdominal cavity is never entered and the iliac vessels can be exposed in the iliac fossa. In females, the round ligament is divided, and in males, the spermatic cord is preserved and mobilized medially. The inferior epigastric vessels can be ligated or preserved. If the patient has had a previous subcostal incision, which implies previous ligation of the superior epigastrics, the inferior epigastrics should be preserved to prevent ischemic necrosis of the anterior abdominal wall. The perivascular lymphatics are ligated to prevent postoperative lymphocele. Standard vascular techniques are used to create anastomoses between the renal vessels and the external iliac vessels using fine monofilament nonabsorbable suture (Fig. 8.2). The kidney is reperfused and hemostasis achieved using sutures, electrocautery, and prothrombotic agents. At the time of reperfusion of the kidney,
the patient is given mannitol as a free radical scavenger and furosemide (Lasix) to promote postreperfusion diuresis.
the patient is given mannitol as a free radical scavenger and furosemide (Lasix) to promote postreperfusion diuresis.
Once the kidney is reperfused and hemostasis achieved, the bladder is exposed at the inferomedial aspect of the wound for creation of a neoureterocystostomy to drain urine into the bladder. The ureterocystic anastomosis is created by incising the posterior aspect of the ureter longitudinally for approximately 1.0 to 1.5 cm. This spatulation allows for the creation of a large ureterocystic anastomosis with absorbable monofilament suture. The bladder wall musculature is then brought together over the top of the anastomosis to create a tunnel, which will minimize reflux of urine into the distal ureter from the bladder. The kidney can be placed on the left side if there is a contraindication to placing the kidney on the right. This is done if previous incisions have been made in the right lower quadrant, if the patient may receive a pancreas transplant in the future, or if the patient has had a previous kidney placed on the right side. The fascia and skin are then closed, and the patient is taken to the recovery room.
When a simultaneous kidney and pancreas transplant is being performed, the pancreas is prepared along with the kidney. During back table preparation of the pancreas, one of the donor’s iliac arterial bifurcations is used to create a vascular conduit to the donor superior mesenteric and splenic arteries in order to provide arterial flow to the pancreas. The portal vein, which will drain the pancreas, is also freed from the surrounding structures in order to allow for the construction of an anastomosis. Once the pancreas is prepared, a midline incision is made in the abdomen. The right iliac artery and vein are exposed through the abdominal cavity. The donor common iliac artery is anastomosed to the recipient’s common iliac artery (Y-graft). If the pancreas will be drained into the systemic venous circulation, then an anastomosis is created between the portal vein and the recipient’s common iliac vein (systemic drainage) (Fig. 8.3). When the pancreas is drained systemically, the head lies in the right lower quadrant of the abdomen, and the tail is directed cephalad in the right pericolic gutter. Alternatively, the superior mesenteric vein can be exposed at the base of the transverse mesocolon and the portal vein drained into the recipient’s portal vein via the superior mesenteric vein (portal drainage) (Fig. 8.4). In the case of portal drainage, the head of the pancreas lies at the base of the transverse mesocolon and the tail lies caudad toward the right lower quadrant. The organ is then reperfused and hemostasis achieved. Portal venous drainage is advocated because the insulin is delivered directly to the liver in a physiologic manner and drainage into the liver is reported to have an immunologic advantage (29, 30, 31).
The past decade of pancreas transplantation has seen a gradual increase in enteric drainage of the pancreas allograft. The use of the bladder to drain the pancreas can be performed when the donor duodenum is compromised. In these situations, the surgeon may feel that the patient would be at increased risk for an enteric leak. However, enteric drainage of the pancreas has been shown to be safe and effective in many centers, and the need to convert 24% of patients from bladder to enteric drainage in a subsequent operation is avoided (30, 31, 32). Once the pancreas is implanted, the kidney is placed in the left lower quadrant within the peritoneum or retroperitoneally by dissecting the peritoneum off the anterior abdominal wall in the left lower quadrant to expose the iliac vessels on the left. We prefer to put the kidney in the retroperitoneum for two reasons: (a) the kidney is held in place by the peritoneum and cannot undergo torsion on its vascular pedicle, and (b) the pancreas and the kidney are in separate compartments should there be a peripancreatic infection or fluid collection.
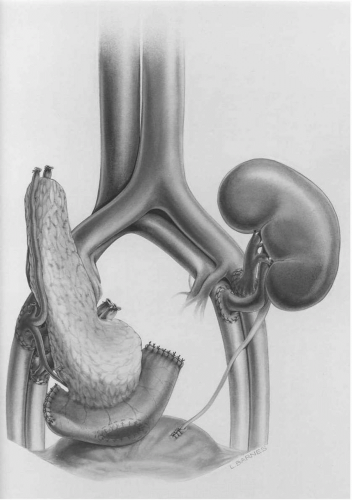 FIG. 8.3. Simultaneous pancreas and kidney transplant with systemic venous drainage and bladder drainage of the exocrine pancreas. |
The most common renal transplants are done with a single renal artery being implanted on the common external or common iliac artery. However, the occurrence of two or more renal arteries is common. When using a cadaver kidney, the two arteries can be anastomosed to the iliac artery using a common patch of aorta. When a living donor has multiple renal arteries, the arteries may be implanted using two separate arteriotomy sites. At times, one or both of the arteries may be too short for the anastomosis to be constructed, or the caliber of the vessel too small to create an
anastomosis. In these cases, a piece of donor gonadal vein or recipient saphenous vein can be used to lengthen the arteries or create a larger caliber conduit for construction of an anastomosis. We advocate using a vein conduit routinely when an artery is less than 3 mm in diameter. Alternatively, an end-to-side anastomosis can be created between the smaller artery and the main renal artery, or two arteries can be opened longitudinally and sutured together to make a common orifice for anastomosis with the iliac artery (33). The presence of multiple renal arteries and the method used to implant them is important to know when ordering postoperative sonograms to evaluate the kidney. The sonographer must know not to end the exam after seeing only one artery. The exam can also confirm flow within the cortex supplied by the different arteries in order to help confirm flow in all implanted vessels. The presence of a small inferior pole renal artery is particularly important since in many cases this vessel provides arterial flow to the ureter.
anastomosis. In these cases, a piece of donor gonadal vein or recipient saphenous vein can be used to lengthen the arteries or create a larger caliber conduit for construction of an anastomosis. We advocate using a vein conduit routinely when an artery is less than 3 mm in diameter. Alternatively, an end-to-side anastomosis can be created between the smaller artery and the main renal artery, or two arteries can be opened longitudinally and sutured together to make a common orifice for anastomosis with the iliac artery (33). The presence of multiple renal arteries and the method used to implant them is important to know when ordering postoperative sonograms to evaluate the kidney. The sonographer must know not to end the exam after seeing only one artery. The exam can also confirm flow within the cortex supplied by the different arteries in order to help confirm flow in all implanted vessels. The presence of a small inferior pole renal artery is particularly important since in many cases this vessel provides arterial flow to the ureter.
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree