Medical Complications of the Eyes, Nasopharynx, Dentition, Oropharynx, and Hearing in the Kidney Transplant Recipient
Daniel J. Salzberg
Donna S. Hanes
Charles B. Cangro
Division of Nephrology, University of Maryland School of Medicine, Baltimore, Maryland 21201
OCCULAR COMPLICATIONS
Vision is our most cherished sense. Clear vision, the ability to recognize one another, to interact with our environment, is central to functioning independently in our world. Because of the essential role of vision in our lives, patients who develop changes in vision seek medical assistance. Transplant recipients are no exception to this rule.
The ocular complications of transplant recipients can be divided into three categories: those resulting from (a) preexisting medical disease (e.g., hypertension, diabetes, or Alport syndrome), (b) the use of immunosuppressive medications, and (c) opportunistic infection.
Preexisting Visual Pathology
The combination of diabetes mellitus and hypertension is the leading cause of end-stage renal disease (ESRD) in the United States (1). Because of better health care, more and more of these patients are living longer, and many are candidates for renal transplantation (2). Before transplantation, during the evaluation process, the visual acuity of potential recipients should be determined. Patients with any type of diabetes should have a full ophthalmologic evaluation. Although poor vision or blindness is not a reason to deny transplantation, special arrangements for medication dispensing and transport to follow-up visits must be established prior to transplantation (3).
After transplantation, during all follow-up encounters, the practitioner should routinely ask each patient about any visual changes, in addition to determining when their last ophthalmologic visit was. Annual follow-up is indicated for any patient with prior ocular pathology, and more frequent follow-up is indicated for subjects with diabetes (4). In addition, the risk for visual disturbances is increased during the first 3 months after a pancreas transplant alone (PTA) or a pancreas after kidney (PAK) transplantation due to the frequent use of warfarin in an effort to prevent thrombosis of the allograft. Early during the postoperative phase, careful attention should be directed at the risk of retinal neovascularization. During the Diabetes Control and Complications Trial, an increase in diabetic retinopathy was observed after rapid normalization of blood sugars in the intensive insulin regimen group (5). Given this result, there is concern that the euglycemic state established after pancreas transplantation might also lead to an increased risk of neovascularization. Therefore, close ophthalmologic follow-up is
necessary after simultaneous pancreas kidney (SPK), PAK or PTA transplantation. Studies to establish the regression of diabetic retinopathy after pancreas transplantation, with or without kidney transplantation, have yielded variable results. To date, there is no incontrovertible evidence that pancreas transplantation leads to a better visual outcome as compared with intensive glycemic control alone (6). The regression of hypertensive retinopathy, by contrast, has been well-documented following renal transplantation and normalization of systemic blood pressure (7).
necessary after simultaneous pancreas kidney (SPK), PAK or PTA transplantation. Studies to establish the regression of diabetic retinopathy after pancreas transplantation, with or without kidney transplantation, have yielded variable results. To date, there is no incontrovertible evidence that pancreas transplantation leads to a better visual outcome as compared with intensive glycemic control alone (6). The regression of hypertensive retinopathy, by contrast, has been well-documented following renal transplantation and normalization of systemic blood pressure (7).
Corneal or conjunctival calcification is a common complication for subjects with ESRD on hemodialysis (8). Tissue injury from abnormal calcium and phosphorus metabolism, in the setting of decreased lubrication and tear formation associated with dialysis, likely contributes to the development of these ocular changes. However, corneal calcifications were not found to be more common after renal transplantation (1).
As transplant recipients survive longer (9), the need to control nondiabetic vasculopathy takes greater importance. Chronic kidney disease (CKD) and dialysis are associated with high cardiovascular morbidity. In transplant patients, a vasculopathy develops which represents a progression of preexisting arteriosclerosis that developed during the course of the patient’s renal failure and time on dialysis (10). After transplant, hyperlipidemia, medications and hypertension may lead to further progression of these vascular changes. This vasculopathy affects the delicate and essential vasculature of the retinal, choroid and optic nerve, which have been shown to deteriorate after transplantation (10).
Given the risk for this vasculopathy, all transplant patients should be screened and treated for lipid abnormalities. Smoking should be strongly discouraged, and all patients should be considered for treatment with aspirin or other antiplatelet adhesion agents (11).
Complications from Immunosuppressant Drugs
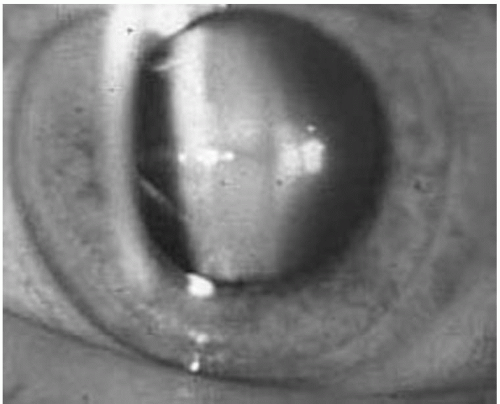
Immunosuppression is required for allograft survival. It was the introduction of 6-mercaptopurine and azathioprine during the 1950s that set the stage for successful allograft transplantation (12). In the 1960s, glucocorticoids were added for induction (13) and maintenance immunosuppression, and high-dose steroids were introduced for the treatment of acute rejection. Unfortunately, ocular side effects are not uncommon. Lens opacification, specifically posterior subcapsular cataract formation (Fig. 35.1), is associated with glucocorticoid use (14). The absence of cataracts before transplantation, the rapid development of them after transplant, and the high incidence of them amongst transplant patients when compared to age-matched controls (45% vs. 10%) (14) are consistent for a role of steroids in cataract formation. The incidence of posterior subcapsular cataracts in patients treated with a prolonged course of steroids has ranged from 10% to 50% with up to 20% of patients developing symptomatic visual impairment (1, 2, 3). Studies have demonstrated a dose-dependent relationship between corticosteroid therapy and cataract formation. Both the total dose of steroids given to a patient (15) and the frequency and amount of high dose pulse steroid exposure has been recognized to be a risk factor for cataract formation (16). Individual susceptibility and genetic factors likely also play a role (15). Cataracts develop more often and become symptomatic earlier posttransplant in elderly and diabetic patients (15).
In most transplant recipients, the prognosis for good vision after cataract extraction and lens implantation is excellent (17). Diabetics who have macular edema are an exception to this rule; these subjects are at higher risk for a poor visual outcome (2,4,5). Therefore, cataract extraction in these subjects should be performed before lens opacification precludes recognition and treatment of macular edema. As stated above, frequent ophthalmologic follow-up is essential in these transplant subjects with diabetes (4).
The pathological changes responsible for lens opacification and cataract formation are poorly understood. Universal theories proposed for cataract formation include: failure of osmotic regulation and localized water accumulation, free radical damage, oxidative changes in lens proteins leading to denaturation, and abnormal aggregation of lens proteins (12).
Little is known about how specific transplant medications might augment these mechanisms. Glucocorticoids and calcineurin inhibitors may alter the amount and type of growth factors reaching the aqueous humor. A role for fibroblast growth factor (FGF), insulin-like growth factor (IGF), platelet-derived growth factor (PDGF) and transforming growth factor beta (TGF-β) have all been proposed (12).
Hampering progress in prevention and treatment of cataracts is the lack of understanding of the underlying mechanism by which they form. Therefore, a variety of different medications and surgical interventions have been tried. Vitamin E administration, in both an animal model and in an in vitro model of steroid-induced cataract formation, has resulted in a decreased incidence and severity of lens opacification (18). It was hypothesized that vitamin E acts as a free radical scavenger, thereby protecting lens proteins from oxidative damage. Despite many claims, no effective
treatment, other than surgical removal of the lens, is currently available for treating cataracts (12).
treatment, other than surgical removal of the lens, is currently available for treating cataracts (12).
Increased intraocular pressure (IOP) is a serious side effect of steroid use, and is reported to occur in 5% to 10% of allograft recipients (3, 4, 5, 6, 7). The route of steroid administration appears to be a factor affecting the incidence of increased IOP, as topical steroids are associated with a higher incidence when compared to systemic steroid therapy. The mechanism by which topical steroids lead to an increase in IOP is by decreasing trabecular aqueous humour outflow. Although systemic steroids also decrease this outflow, they have the additional effect of decreasing the formation of the aqueous humor, resulting in a lower incidence of increased IOP (3). Increases in IOP are asymptomatic and can only be detected during ophthalmologic exam using a tonometer. If left untreated, these increases can lead to visual field defects, atrophy of the optic nerve, and blindness. In transplant patients, increased IOP can be treated with topical and systemic medicines, laser therapy, and surgery interventions. However, topical drops that result in miosis may decrease the visual acuity in patients who also have cataracts.
Ocular Infections in the Immunocompromised Host
Opportunistic infection of the eye occurs at a frequency of approximately 2% in immunosuppressed patients (17).
Conjunctivitis
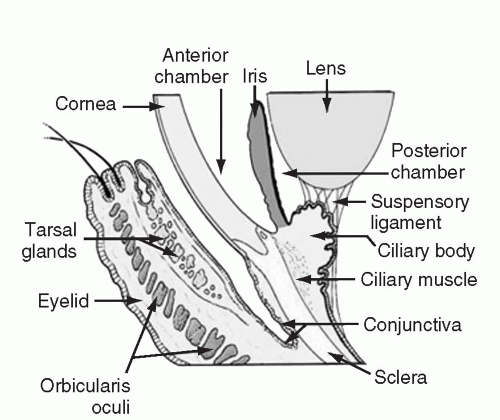
Conjunctivitis, defined as an inflammation of the conjunctiva, is a common complaint in the general population, and transplant patients are no different. The conjunctiva is comprised of a mucous membrane that lines the inside surface of the eyelid and covers the globe up to the junction of the sclera and cornea (Fig. 35.2). It is generally transparent and composed of two layers, a non-keratinized epithelium and a highly vascular substantia propria. The substantia propria is the site of significant immunologic activity. When inflamed, the conjunctiva looks red to the unaided eye. On closer examination the redness is recognizable as distinct small blood vessels. All cases of conjunctivitis are manifest by red eye; however, not all red eyes are the results of conjunctivitis.
Red eye is a common presenting complaint. When evaluating a red eye, it is imperative to rule out sight threatening pathologic processes that require immediate referral to an ophthalmologist (15).
Evaluation of a patient with “red eye” must include testing of visual acuity as well as a penlight examination. If visual impairment is discovered, a more serious condition other than conjunctivitis must be considered, such as infectious keratitis, iritis or acute angle closure glaucoma. All of these conditions require, expeditious ophthalmologic evaluation. On penlight exam of the eye, a fixed pupil in middilatation is typical of acute angle glaucoma, and a pinpoint pupil suggests possible corneal abrasions, infectious keratitis or iritis. A white spot or opacity on the cornea is highly suggestive of infectious keratitis and requires emergent referral. If the patient complains of a “foreign body” sensation and is unable to keep the eye open, then an active corneal process should be suspected and urgent referral to an ophthalmologist is indicated. Patients with subjective complaints of “grit or sand” in the eye, who do not complain of an inability to open or keep the eye open, may not have a corneal process. Indications for a transplant patient to be immediately referred to an ophthalmologist include traumatic facial or eye injuries, severe photophobia, and eye complaints in subjects who wear contact lenses due to the high risk for ocular infections.
Patients often present complaining of watery eyes and waking up with a crusty “pus like” discharge. This is frequently due to self-limited processes such as viral conjunctivitis, allergic reactions, dry eyes, or inflammation of the sebaceous glands of the eyelid, i.e., a stye. Patients with these benign processes do not manifest photophobia. They may have associated allergic or viral symptoms, such as rhinorrhea, cough, or pharyngitis. Bacterial conjunctivitis is associated with an opaque discharge that persists throughout the day and is not associated without photophobia, a decrease in visual acuity or a foreign body sensation. Superficial bacterial processes may be initially treated with a topical antibiotic preparation such as erythromycin ophthalmic ointment or sulfa ophthalmic drops (16). Any change in the subjective or objective complaints, however, should prompt referral to an ophthalmologist.
Finally, either a hypopyon (defined as a layer of white cells in the anterior chamber) or a hyphema (defined as a layer of red cells) requires urgent referral to an ophthalmologist. Hypopyon indicates a serious infectious keratitis or endophthalmitis while a hyphema is suggestive of significant trauma to the eye.
Endophthalmitis
Endophthalmitis refers to infection within the eye. Because the vitreous is in direct contact with the retina, endophthalmitis is a sight-threatening infection and requires immediate attention (Fig. 35.3).
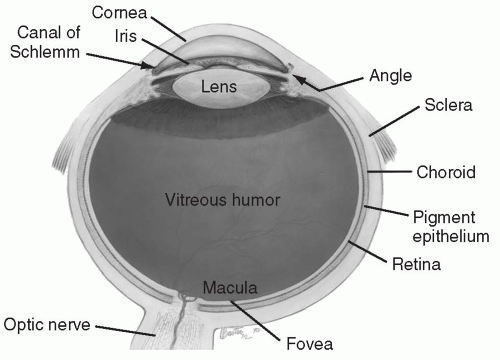 FIG. 35.3. Note proximity of vitreous humor to retina.
Stay updated, free articles. Join our Telegram channel
Full access? Get Clinical Tree
 Get Clinical Tree app for offline access
Get Clinical Tree app for offline access

|