Fig. 8.1
Computed tomography scan of a locally advanced tumor (a) prior to chemotherapy and (b) following chemotherapy and SBRT to 33 Gy in 5 fractions. Patient then underwent a successful margin- and node-negative resection in which only scattered microscopic foci of adenocarcinoma (a near-pathologic complete response) was found
In this chapter, we will explore the published data, including that of retrospective and prospective studies in the field of SBRT for pancreatic cancer. The opportunities and challenges in the utilization of this technique, including appropriate patient selection and treatment methodology, will be discussed.
Resectability in Borderline Resectable and Locally Advanced Pancreatic Cancer
In pancreatic cancer , surgical resectability is considered paramount in achieving a cure. To determine whether a tumor is resectable, careful consideration of arterial and venous involvement—the superior mesenteric artery (SMA), celiac axis, common hepatic artery (CHA), superior mesenteric vein (SMV), and portal vein (PV) specifically—is taken into account. While the nomenclature defining surgical resectability has remained fairly constant for years, the definition of borderline resectable disease was recently formalized by a consensus group from the Americas Hepato-Pancreatico-Biliary Association (AHPBA), Society of Surgical Oncology (SSO), and Society for Surgery of the Alimentary Tract (SSAT) [8]. These criteria are often referred to as the Consensus or Callery guidelines and have been reproduced in Table 8.1. The criteria adopted by the National Comprehensive Cancer Network (NCCN) are listed in Table 8.2 [2]. A more refined definition of borderline resectable tumors, classically a difficult subgroup to define, is noted in Table 8.3 [9]. The definition listed in Table 8.3 provides specific criteria used in the Intergroup trial (A021101) testing neoadjuvant FOLFIRINOX followed by 50.4 Gy of external beam radiation and capecitabine in patients with borderline resectable pancreatic cancer [9]. Due to the heterogeneous definitions of resectability, careful consideration of these criteria and the involved vasculature is necessary to compare clinical outcomes among populations involving patients with borderline resectable and locally advanced pancreatic cancer. Standardization of resectability in pancreatic cancer is essential.
Resectability status | Criteria | Median survival |
|---|---|---|
Resectable | No distant metastases | 20–24 months |
No radiographic evidence of SMV and portal vein abutment, distortion, tumor thrombus, or encasement | ||
Clear fat planes around the celiac axis, hepatic artery, and SMA | ||
Borderline resectable | No distant metastases | Resected: ~20 months |
Venous involvement of the SMV/portal vein demonstrating tumor abutment with or without impingement and narrowing of the lumen, encasement of the SMV/portal vein but without encasement of the nearby arteries, or short segment venous occlusion resulting from either tumor thrombus or encasement but with suitable vessel proximal and distal to the area of vessel involvement, allowing for safe resection and reconstruction | ||
GDA encasement up to the hepatic artery with either short segment encasement or direct abutment of the hepatic artery without extension to the celiac axis | ||
Unresected: ~11 months | ||
Tumor abutment of the SMA not to exceed >180° of the circumference of the vessel wall | ||
Locally advanced | HEAD: No distant metastases; SMA encasement exceeding >180° or any celiac axis abutment; unreconstructible SMA/portal vein occlusion/encasement; extensive hepatic artery involvement; aortic invasion or encasement | 9–15 months |
BODY: No distant metastases; SMA or celiac axis encasement >180°; unreconstructible SMV/portal occlusion; aortic invasion | ||
TAIL: No distant metastases; SMA or celiac axis encasement >180° | ||
ALL: Metastases to lymph node beyond the field of resection | ||
Metastatic | Any presence of distant metastases | 4–6 months |
Stage | Arterial | Venous |
|---|---|---|
Resectable | Clear fat planes around celiac axis, superior mesenteric artery, and hepatic artery | No superior mesenteric vein/portal vein distortion |
Borderline resectable | Gastroduodenal artery encasement up to the hepatic artery with either short segment encasement or direct abutment of the hepatic artery without extension to the celiac axis. Tumor abutment of the superior mesenteric artery not to exceed greater than 180° | Venous involvement of the superior mesenteric vein or portal vein with distortion or narrowing of the vein or occlusion of the vein with suitable vessel proximal and distal, allowing for safe resection and placement |
Unresectable | Aortic invasion or encasement. Based on tumor location: pancreatic head—more 180° encasement, any celiac axis abutment, inferior vena cava; pancreatic body/tail—superior mesenteric artery or celiac axis encasement greater than 180° | Unreconstructable superior mesenteric vein/portal vein occlusion |
Vessel | Tumor involvement |
|---|---|
Superior mesenteric vein–portal vein | Interface between tumor and vessel measuring 180° or greater of the circumference of the vessel wall, and/or reconstructable occlusion |
Superior mesenteric artery | Interface between tumor and vessel measuring less than 180° of the circumference of the vessel wall |
Common hepatic artery | Reconstructable, short-segment interface between tumor and vessel of any degree |
Celiac trunk | Interface between tumor and vessel measuring less than 180° of the circumference of the vessel wall |
In general, patients with LAPC are considered unsuitable candidates for upfront surgery, in part due to the morbidity and mortality risk associated with vasculature resection [10]. Additionally, the decision to resect a tumor with a high likelihood of a positive margin at the site of vascular involvement is suboptimal as the survival of patients with a microscopically (R1) or grossly (R2) positive margin has been shown to be significantly inferior to patients resected to a negative (R0) margin [10, 11]. The standard-of-care in these patients is most often upfront chemotherapy alone or CRT. The goal of this therapy is to optimally downsize (or, if possible, sterilize) the tumor to allow for surgical resection and increase the likelihood of improved pathologic outcomes (i.e., margin- and node-negative resection, pathologic complete response). In fact, a recent study has suggested promising outcomes in 40 patients with BRPC or LAPC who underwent neoadjuvant FOLFIRINOX (5-fluorouracil, irinotecan, leucovorin, and oxaliplatin) therapy. Of these 40 patients, 30 (75 %) received radiation therapy: 24 received 50.4 Gy CRT and 5-fluorouracil (5-FU), 10 of which also received a 7–12 Gy intraoperative radiation therapy (IORT) boost, and 6 received proton beam therapy with charged particles. On final pathology, the patients who received neoadjuvant therapy had a significant decrease in lymph node positivity (35 % vs. 79 %) and perineural invasion (72 % vs. 95 %) in comparison with 87 patients who underwent upfront surgery. Furthermore, the neoadjuvant patients achieved margin-negative and node-negative resection rates of 92 % and 65 %, respectively.
Unpublished data exploring neoadjuvant SBRT in borderline and locally advanced patients at Johns Hopkins University. Among 80 resected patients with BRPC or LAPC, 33 received neoadjuvant chemotherapy alone and 47 received induction chemotherapy followed by SBRT. FOLFIRINOX-based chemotherapy was administered to 63 and 45 % of the SBRT group and chemotherapy group, respectively. The majority (57 %) of SBRT patients were deemed unresectable while only 24 % in the chemotherapy alone group had LAPC (p = 0.009). Pancreaticoduodenectomy was performed in 68 % of patients who underwent SBRT vs. 85 % of patients who received chemotherapy (p = NS). In the SBRT group, the R0 resection rate was 85 % in BRPC and 89 % in LAPC vs. 48 % in BRPC and 63 % in LAPC patients in the chemotherapy group (p = NS). Node-negative resections were achieved in 72 % of patients who received SBRT (60 % in BRPC, 81 % in LAPC) vs. 42 % of patients who received chemotherapy alone (40 % in BRPC, 50 % in LAPC) (p = NS). The pathologic complete response rate was 13 % in the SBRT group (10 % in BRPC, 15 % in LAPC) vs. 3 % in the chemo group (0 % in BRPC, 13 % in LAPC) (p = NS). The near-pathologic complete response rate, defined as microscopic foci of single cells or groups of single cells of adenocarcinoma, was 28 % in the SBRT group (25 % in BRPC, 30 % in LAPC) vs. 12 % in the chemotherapy group (12 % in BRPC, 13 % in LAPC) (p = NS). Figures 8.2 and 8.3 demonstrate the extensive treatment effect seen macroscopically (Fig. 8.2) and microscopically (Fig. 8.3) in patients who underwent neoadjuvant SBRT. Further follow-up data is underway to determine the impact of these pathologic outcomes on survival.
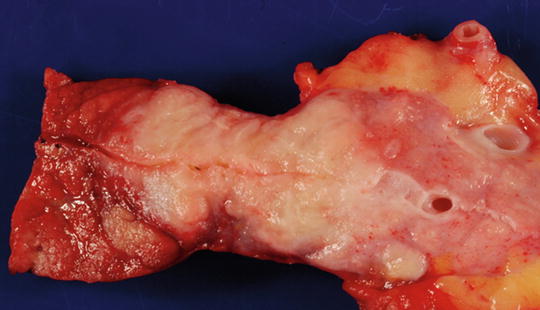
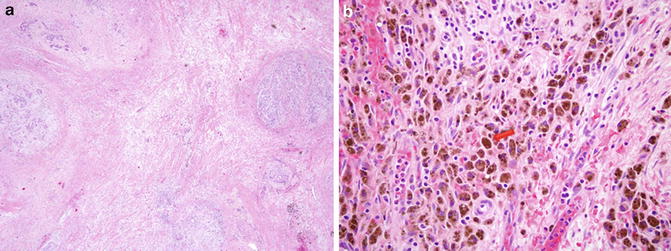

Fig. 8.2
Resected bivalve specimen has been sliced along the pancreatic duct. The pancreas (the left side) looks hyperemic. The tumor is located in the center. The upstream pancreas is to the right (towards the spleen). The dilated pancreatic duct and the stroma appear to be edematous. Courtesy of Ralph H. Hruban

Fig. 8.3
Microscopic evidence of (a) extensive treatment effect observed following pancreas SBRT to a tumor that was measured to be 3.8 cm in size, and (b) the presence of hemosiderin-laden macrophages (brown cells), inflammatory cells that are suggestive of a reactive process following therapy
Standard Treatment for Borderline Resectable and Locally Advanced Pancreatic Cancer
The morbidity and potential mortality associated with surgical resection of BRPC and LAPC implies that CRT or chemotherapy alone is the only viable option for cure in these patients [12]. Despite the completion of multiple studies on this topic, no consensus regarding the optimal course of management exists. The most recent NCCN clinical practice guidelines recommend enrollment onto a clinical trial as the first-line option [2]. In patients with good performance status, multi-agent chemotherapy followed by CRT is considered appropriate.
Data supporting the above approach are derived from decades of clinical trials dating back to the 1980s [4, 5, 13–17]. Table 8.4 presents a selection of the clinical trials which have investigated the role of standard fractionated radiation in LAPC. As is evidenced by the table, the survival of patients has not progressed dramatically despite the numerous advances in chemotherapy agents and radiation technology in the last three decades.
Table 8.4
Selected studies of locally advanced pancreatic cancer
Study | Number | Treatments | Median survival (months) | P value |
|---|---|---|---|---|
GITSG Moertel [13] | 194 | 60 Gy vs. 60 Gy + 5FU (bolus) or 40 Gy + 5FU (B) | 5.7 vs. 10.1 or 10.6 | <0.01 |
GITSG [14] | 43 | Streptozocin, MMC, 5FU vs. 54Gy + 5FU (bolus) → Streptozocin, MMC, 5FU | 8 vs. 10.5 | <0.02 |
ECOG Klaassen [15] | 91 | 5FU (bolus) vs. 40 Gy + 5FU (bolus) → 5FU | 8.2 vs. 8.3 | ns |
FFCD/SFRO Chauffert [5] | 119 | Gem vs. 60 Gy + 5FU (continuous infusion) + Cis → Gem | 13 vs. 8.6 | 0.03 |
ECOG Loehrer [4] | 74 | Gem vs. 50.4 Gy + Gem → Gem | 9.2 vs. 11 | 0.04 |
GERCOR Huguet [16]a | 181 | Gem-based Chemo vs. Gem-based Chemo → Chemorad | 1.7 vs. 15 | 0.0009 |
MDACC Krishnan [17]a | 323 | Chemorad vs. Gem-based Chemo → Chemorad | 8.5 vs. 11.9 | <0.001 |
The most significant debate in the appropriate management of patients with BRPC and LAPC centers on the role of radiation in this disease. Some studies have demonstrated a survival decrement with the application of radiation therapy in this patient population. However, these studies suffer from major drawbacks, including poor radiation quality assurance, excess radiation dose, unclear dose constraints for adjacent critical structures, and the use of “split-course” radiation in which a 2-week treatment break is part of the planned course of treatment. Other studies have shown a potential benefit for radiotherapy [4]. However, a major criticism of all these data is the utilization of outdated or ineffective chemotherapy.
A more modern approach to the treatment of this disease has been to use combination chemotherapy with either FOLFIRINOX or gemcitabine with nab-paclitaxel [18–20]. These two combination chemotherapeutic regimens have demonstrated a survival benefit in comparison to gemcitabine alone, albeit in the metastatic setting. In BRPC and LAPC, the current NCCN guidelines recommend either single-agent gemcitabine or combination chemotherapy, with CRT preferred following a course of initial chemotherapy. SBRT is listed as an option, though its use is encouraged as part of enrollment on a clinical trial [2].
Stereotactic Body Radiation Therapy
Traditional radiotherapy has been delivered in small daily fractions to take advantage of the ability of normal human tissue to repair radiation more quickly than tumor tissue. This “therapeutic window” is particularly critical in anatomical locations prone to severe, irreparable radiation damage [7]. One of the dangers of using high-dose-per-fraction radiation is the risk of overwhelming the therapeutic window and damaging sensitive adjacent normal tissues without precise targeting of the tumor [21, 22]. However, the development of advanced radiotherapy techniques in the last two decades has dramatically changed the landscape of radiation oncology [6].
SBRT is defined as the use of intensity modulation, image guidance, tumor motion control, and stereotactic targeting to deliver a high dose of radiation to the tumor in five or less fractions [6]. Each of the aforementioned techniques and technological developments contributed to the ability to use this type of treatment. Image guidance ensures that the tumor and/or fiducial or stent is visualized at the time of each treatment, allowing for reduced treatment margins (thereby reducing normal tissue exposure). Whereas treatment margins had historically been measured in centimeters, the use of this technology has reduced these margins to only a few millimeters (mm) [6].
SBRT was first used to treat intracranial neoplasms [23]. Later, this was expanded to extracranial sites, particularly with early stage lung cancer, demonstrating outstanding local control, virtually absent acute (<3 months) toxicity, and minimal chronic (>3 months) toxicity [24]. By nature of its “parallel” normal tissue unit arrangement, lung tissue benefits from being able to receive an ablative dose to one region without compromising the overall function of the organ. In contrast, the perceived risk of using SBRT in locations abutting normal tissues with a “serial” arrangement of normal tissues, including the small bowel and stomach as seen with the pancreas, is more concerning. Consequently, SBRT to areas within the abdomen and pelvis have been adopted with much more caution [6]. Without a firm understanding of the dose constraints of these sensitive organs at risk (OARs), practitioners have been hesitant to use ablative doses of radiation in this region. As data has emerged from groups that have utilized this approach, a stronger understanding of the dose tolerance of the small bowel and stomach has led to the widespread adoption of SBRT in the infradiaphragmatic space [25]. An analysis of patterns of care of radiation delivery from 39 centers in the United States indicates that the use of SBRT for pancreatic cancer is increasing, but still represents a relatively small absolute value [25].
In the following sections, the clinical results, toxicities, and techniques for the safe and effective utilization of pancreas SBRT are described.
Clinical Trials Utilizing SBRT for Borderline Resectable and Locally Advanced Pancreatic Cancer
In the last decade, retrospective reports and prospective clinical trials have supported the use of pancreas SBRT as a potent method for providing excellent tumor control, increasing resectability rates, and improving surgical outcomes in patients with BRPC and LAPC (Table 8.5) [26–40]. However, heterogeneity in selection criteria, patient immobilization technique, radiation dose, radiation planning techniques, and radiation delivery devices limit direct comparisons between these studies.
Table 8.5
Prospective and retrospective investigations in stereotactic radiotherapy for pancreatic cancer
Author | Prosp | Retro | BR | LA | Total | LINAC/CK | DPF (Gy) | Fractions | Total dose (Gy) | 1 year LC | PFS (months) | OS (months) | 1 year OS | 2 year OS |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
Koong [26] | I | 0 | 15 | 15 | CK | 15–25 | 1 | 15–25 | 100 % | 11 | ||||
Hoyer [27] | I | 0 | 22 | 22 | LINAC | 15 | 3 | 45 | 57 % | 4.8 | 5.4 | 5 % | ||
Koong [28] | I/II | 0 | 16 | 16 | CK | 25 | 1 | 25 | 94 % | 4 | 11.4 | 15 % | ||
Chang [29] | X | 0 | 45 | 77 | CK | 25 | 1 | 25 | 87 % | 26 % @ 6 months | 11.9 | 21 % | ||
Polistina [30] | I | 0 | 23 | 23 | CK | 10 | 3 | 30 | 83 % | 7.3 | 10.6 | 39 % | 0 % | |
Schellenberg [31] | II | 0 | 20 | 20 | CK | 25 | 1 | 25 | 75 % | 9.2 | 11.8 | 50 % | 20 % | |
Mahadevan [32] | X | 0 | 36 | 36 | CK | 8–12 | 3 | 24–36 | 78 % | 9.6 | 14.3 | |||
Mahadevan [33] | X | 0 | 47 | 47 | CK | 8–12 | 3 | 24–36 | 85 % | 15 | 20 | |||
Chuong [34] | X | 57 | 16 | 73 | LINAC | 7–10 | 5 | 35–50 | 86 % | 9.7 BR/ 9.8 LA | 16.4/15 | 72.2/68.1 | ||
Tozzi [35] | I/II | 21 LA, 9 recurrent | 30 | LINAC | 7.5 | 6 | 45 | 85 % | 8 | 11 | 47 % | |||
Kim [36] | X | 10 | 7 | 17 | LINAC | 8–24 | 1–3 | 24–36 | 53 % | 6.3 | 7.6a | 35 % | ||
Rajagopalan [37] | X | 7 | 5 | 12 | both | 10–24 | 1–3 | 25–36 | 6.3 | 10.9 | 92 % | 64 % | ||
Gurka [38] | X | 6 | 28 | 34 | CK | 5–6 | 5 | 25–30 | 79 % | 6.8 | 12.3a | |||
Moningi [39] | X | 14 | 74 | 88 | LINAC | 5–6.6 | 5 | 25–33 | LPFS 13.9 | 9.8 | 18.4 | |||
Herman [40] | II | 0 | 49 | 49
Stay updated, free articles. Join our Telegram channel
Full access? Get Clinical Tree
 Get Clinical Tree app for offline access
Get Clinical Tree app for offline access

| ||||||||||




