CHAPTER 97 Small Intestinal Motor and Sensory Function and Dysfunction
ANATOMY
The small intestine is approximately 3 to 7 meters long and extends from the duodenal side of the pylorus to the ileocecal valve. It is divided into three regions—duodenum, jejunum, and ileum—based on structural and functional considerations. Although some structural and functional differences exist among these three regions, they exhibit similar motor characteristics. At each end of the small intestine, however, physiologic sphincters—the pylorus and the ileocecal valve—have distinctly different motor patterns that give them the ability to act as controllers of flow between the antrum and duodenum and between the ileum and colon, respectively. The motor function of the pylorus is discussed in Chapter 48, the ileocecal region is discussed in Chapter 98, and general anatomy is discussed in Chapter 96. The duodenum is a fixed, largely retroperitoneal structure located in the upper abdomen, and the distal ileum generally is anchored in the right iliac fossa by its attachment to the cecum. Except for these regions, the small intestine is mobile within the peritoneal cavity.
KEY ELEMENTS IN NORMAL SMALL INTESTINAL MOTOR AND SENSORY FUNCTION
SMOOTH MUSCLE
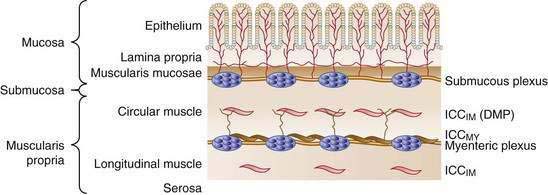
The wall of the small intestine comprises the mucosa, consisting of the epithelium and lamina propria; submucosa; muscular layer (muscularis); and serosa (Fig. 97-1). The muscularis is composed of inner circular and outer longitudinal layers of smooth muscle, which are present in continuity along the length of the small intestine. Contractions within these layers are responsible for gross small intestinal motility. A much smaller additional muscular layer, the muscularis mucosae, is present between the mucosa and the submucosa and plays a role in mucosal or villus motility1 but does not contribute to gross motility and is not considered further in this chapter.
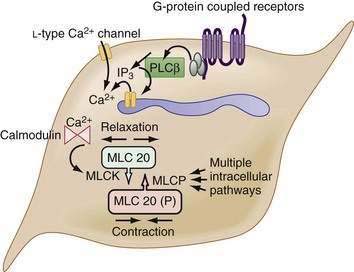
The myocytes themselves are spindle-shaped cells that derive their contractile properties from specialized cytoplasmic filaments and from the attachment of these filaments to cytoskeletal elements. On electron microscopy, condensations of electron-dense, amorphous material are noted around the inner aspect of the cell membrane (dense bands) and throughout the cytoplasm (dense bodies). The contractile filaments—actin and myosin—are arranged in a fashion similar to that in skeletal muscle and insert onto the dense bands and bodies approximately in parallel with the long axis of the cell. Thus, when the contractile filaments are activated to slide over each other, cell shortening results. Most of the Ca2+ required for activating the contractile apparatus enters the cells via L-type Ca2+ channels (Fig. 97-2). Ca2+ entry also can be supplemented to a varying extent by release of Ca2+ from the sarcoplasmic reticulum membrane via IP3 receptor–operated Ca2+ channels. IP3 is generated by phospholipase C, which in turn is activated by G-proteins, coupled to receptors for excitatory transmitters (G-protein–coupled receptors).
The increased cytoplasmic Ca2+ binds to the Ca2+ binding protein calmodulin, enabling it to activate myosin light chain kinase, which phosphorylates the 20 kD light chain of myosin (MLC20). Phosphorylation of MLC20 facilitates actin binding to myosin and initiates cross-bridge cycling and development of mechanical force. Phosphorylation of MLC20 is reduced by MLC phosphatase. Dephosphorylation of MLC20 reduces cross-bridge cycling and leads to muscle relaxation. The dephosphorylation process is under a complex system of hierarchical control, which is important in setting the gain of smooth muscle contractility.2
INTERSTITIAL CELLS OF CAJAL
Interstitial cells of Cajal (ICC) are specialized cells within the smooth muscle layer that are vital for normal small intestinal motor function. ICC are pleomorphic mesenchymal cells that form an interconnecting network via long, tapering cytoplasmic processes. ICC lie in close proximity to both nerve axons and myocytes, with which they form electrical gap junctions.3 ICC serve two roles in control of small intestinal motility: first, they act as pacemakers generating the electrical slow wave that determines the basic rhythmicity of small intestinal contractions4; second, they transduce both inhibitory and excitatory neural signals to the myocytes5 and thus can vary the myocyte membrane potential and, in turn, contractile activity. This transduction occurs because ICC are interposed functionally between nerve terminals and the smooth muscle that the nerves supply. The neuroeffector junctions of the small intestine are not just simple contacts between nerve terminals and smooth muscle cells; they are contacts between enteric nerve terminals and ICC, and from there with myocytes by means of electrical gap junctions. Thus, effective neurotransmission results from the activation of specific sets of receptors on ICC, rather than by direct action on smooth muscle cells.
Cells of the ICCMY population form a dense, electrically coupled network within the intermuscular space at the level of the myenteric plexus between the circular and longitudinal muscle layers. ICCMY are the pacemaker cells in the small intestine that trigger generation of slow waves in the smooth muscle. These cells possess a specialized mechanism that uses their oxidative metabolism to generate an inward (pacemaker) current, resulting from the flow of cations through nonselective cation channels in the plasma membrane. A primary pacemaker initiates slow waves. This depolarization from the primary event then entrains the spontaneous activity of other ICC within the network. This sequence results in a propagation-like phenomenon by which slow waves spread, without decrement, through the ICC network by means of gap junctions. A specialized type of ICCMY line the septa (ICCSEP) between circular muscle bundles; these cells form a crucial conduction pathway for spreading excitation deep into muscle bundles of the human jejunum, which is necessary for the motor patterns underlying mixing.6
ICC, in general, play broadly similar roles in the small intestine and colon, and the reader is referred to Chapter 98 for a discussion of their roles in the large bowel (see also Fig. 98-2), as well as recent reviews by Sanders, Ward, and their colleagues.4,5 Absence or inactivity of ICC has been implicated in a number of clinical disorders that manifest as disturbed intestinal motility (see Chapter 20).
NEURONS
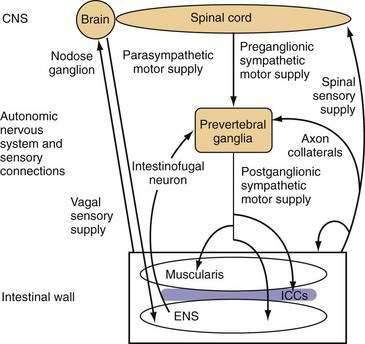
The small intestine is richly innervated with both extrinsic and intrinsic neurons. Intrinsic neurons have their cell bodies within the wall of the small intestine and constitute the ENS. These intrinsic neurons greatly outnumber the neurons of the extrinsic supply, which have their cell bodies outside the gut wall, but they have projections that end within the intestinal wall. Extrinsic neurons can be classified anatomically according to the location of their cell bodies and the route along which their projections travel. Extrinsic motor neurons belong to the ANS and connect the central nervous system (CNS) with the ENS and, from there, the small intestinal smooth muscle through the ICC. Some extrinsic motor neurons terminate directly in the muscle layers. Extrinsic sensory neurons from the small intestine do not belong to the ANS and are classified as spinal or vagal, depending on the route they follow to the CNS (Fig. 97-3).
Neurons supplying the intestine are designated either afferent or efferent, depending on the direction in which they conduct information. By convention, information is conducted centrally by afferent neurons and peripherally by efferent neurons. Thus, the term afferent in regard to neural supply is used to describe pathways conducting information that is detected in the intestine; in most texts “afferent” is interchangeable with the “sensory,” although most sensory information from the small intestine is not perceived at a conscious level. The terms efferent and motor in regard to neural supply are used to describe pathways conducting signals toward the effector small intestinal smooth muscle. Although the importance of motor innervation for motility is self-evident, the pivotal role of afferent function in determining motor responses has been less well appreciated. The importance of the extrinsic afferent innervation is emphasized by the observation that at least 80% of vagal fibers are afferent.7
Intrinsic Neurons
ENS elements of the small intestine can be subdivided into three major functional groups: primary sensory (afferent) neurons, motor (efferent) neurons, and interneurons. Other categories of neurons, including secretomotor and vasomotor neurons and motor neurons to endocrine cells, are recognized, but they are not considered further in this chapter. Many distinct groups of enteric neurons are now well characterized both structurally and functionally and are reviewed in detail elsewhere.8,9
Afferent Supply
The primary afferent neurons of the ENS morphologically are Dogiel type II neurons (neurons with numerous processes).10 Intrinsic primary afferent neurons that respond to mucosal chemical stimuli have their cell bodies in the myenteric plexus, and they project axons toward the mucosa. The myenteric plexus also contains the cell bodies of intrinsic afferent neurons that discharge in response to mechanical stimulation of the muscle layer induced by muscle activity or stretch. Intrinsic afferent neurons that respond to mechanical stimulation of the mucosa also are believed to exist, based on enteric reflexes seen in extrinsically denervated preparations. The cell bodies and processes of these neurons have not yet been identified definitively, although available evidence is consistent with the presence of their cell bodies in the submucosal ganglia.10
Intrinsic sensory neurons synapse in the intramural plexuses with intrinsic motor neurons and interneurons, which they excite mainly by release of acetylcholine and substance P. A more detailed account of the function and role of intrinsic afferent neurons can be found in a review by Furness and coworkers.10
Observations indicate that other classes of enteric neurons also respond to mechanosensory stimuli, suggesting that the ENS behaves as a sensorimotor network rather than as separate components.11
Efferent Supply
The axons of the intrinsic motor neurons that supply small intestinal smooth muscle exit the intramural ganglia and enter either the circular or the longitudinal muscle layer, where they pass in close proximity to both the myocytes and ICC. No specific neuromuscular junctions are present in small intestinal smooth muscle as in skeletal muscle, although the multiple varicosities along the motor axons probably represent specialized areas of neurotransmission. The motor axons discharge along their length, potentially activating large numbers of myocytes through ICC but possibly also directly activating them. The lack of exclusive, specific neuromuscular junctions, the electrical gap junctions among myocytes, and the overlap of innervation of myocytes from more than one motor axon mean that functionally discrete motor units in the intestinal smooth muscle do not appear to exist, in contrast with skeletal muscle. The ENS motor supply itself is both inhibitory and excitatory, and intrinsic motor neurons generally contain both a fast and a slow neurotransmitter. The predominant excitatory transmitters are acetylcholine (fast) and substance P (slow), and the predominant inhibitory transmitters are nitric oxide (fast), vasoactive intestinal polypeptide (VIP) (slow), adenosine triphosphate (ATP) (fast), and the nucleotide β-nicotinamide adenine dinucleotide (β-NAD).12
Interneurons
Interneurons connect ENS neurons of the same class or of different classes with one another. They permit local communication within limited lengths of intestinal wall (measured in millimeters or centimeters) and are implicated in simple local responses by means of release of acetylcholine or nitric oxide, depending on their oral or aboral direction of projection. Some evidence also suggests the presence of connections within the intestinal wall along greater distances, but these neural pathways are not well defined. These connections may be provided anatomically by the ENS or by connections between the ENS and ANS. Interneurons that play an additional sensory role have been identified.11
A special type of interneuron, the intestinofugal neuron, may be important for controlling local reflexes. Intestinofugal neurons have cell bodies within the myenteric plexus. These cell bodies receive input from several local enteric neurons and project to the prevertebral ganglia, where they synapse with sympathetic motor neurons (see Fig. 97-3).13
Extrinsic Neurons
Afferent Supply
The small intestine is innervated by vagal and spinal extrinsic afferents. The pathway of small intestinal vagal afferent innervation is relatively straightforward. The vagal afferent neurons have endings in the intestinal wall and cell bodies within the nodose and jugular ganglia, which deliver input directly to the brainstem. Spinal afferent fibers travel along perivascular nerves to the prevertebral ganglia, where neurons do not end but might give off axon collaterals that synapse on postganglionic sympathetic motor neurons; these fibers then pass into the thoracic spinal cord along the splanchnic nerves. Spinal afferent neurons have their cell bodies throughout the thoracic dorsal root ganglia and enter the spinal cord through the dorsal roots; they synapse mainly on neurons of the superficial laminae of the spinal gray matter. These neurons, in turn, can send projections to numerous areas of the brain involved in sensation and pain control. Spinal afferent neurons also can give off axon collaterals closer to the intestinal wall, which synapse on components of the ENS, blood vessels, smooth muscle, or secretory elements (see Fig. 97-3). The different stimulus response profiles of vagal and splanchnic mechanoreceptors are generally interpreted as evidence that vagal afferents subserve physiologic regulation, and splanchnic afferents mediate pain.14–16
Functionally, three distinct and characteristic patterns of terminal distribution can be identified within the intestinal wall. Extraluminal afferent fibers have responsive endings on blood vessels in the outer, serosal layer and in the mesenteric connections. Muscular afferents form endings either in the muscle layers or in the myenteric plexus.17 Mucosal afferents form endings in the lamina propria, where they are positioned to detect substances absorbed across the mucosal epithelium or released from epithelial and subepithelial cells, including enterochromaffin and immunocompetent cells.17
These three different populations of afferent endings have different sensory modalities, responding to both mechanical and chemical stimulation.14,18 Serosal and mesenteric afferents are found mainly in the splanchnic innervation and are activated by distortion of the intestine and its attachments; they do not normally signal distention or contraction of the bowel wall unless it is strong enough to cause distortion of the outer layers. Serosal and mesenteric receptors also commonly show evidence of chemosensitivity. This observation hints at potential responsiveness to circulating or locally released factors, especially in view of the localization of these receptors on or near blood vessels.19
Muscular afferents respond to distention and contraction with lower thresholds for activation, and they reach maximal responses within levels of distention that are encountered normally during digestion. Muscular afferents show maintained responses to distention of the small intestine and signal each contractile event, giving rise to the term in-series tension receptors. Nerve tracing studies have identified vagal afferent terminals in the longitudinal and circular muscle layers described as intramuscular arrays (IMAs), consisting of several long (up to a few millimeters) and rather straight axons running parallel to the respective muscle layer and connected by oblique or right-angled short connecting branches.17,20 IMAs were proposed to be the in-series tension receptor endings, possibly responding to both passive stretch and active contraction of the muscle, although direct evidence for this proposal is currently lacking.
Vagal afferent terminals surrounding the myenteric plexus throughout the gastrointestinal tract have been described as intraganglionic laminar endings (IGLEs). These endings are in intimate contact with the connective tissue capsule and enteric glial cells surrounding the myenteric ganglia, and they have been hypothesized to detect mechanical shearing forces between the orthogonal muscle layers. Evidence for such a mechanosensory function of IGLEs has been elaborated by mapping the receptive field of vagal afferent endings in the esophagus, stomach, and large intestine, showing morphologically that individual hot spots of mechanosensitivity correspond with single IGLEs.21 Functional evidence exists for muscular afferents in both the vagal and the spinal innervation, but the appearance of spinal distention-sensitive afferents in the small intestine is yet to be determined. It is likely to be distinct from that of vagal afferents due to their higher thresholds for distention.22
Small intestinal mucosal afferents have been found in the vagal supply, but their existence in the spinal supply can be inferred only from the fact that they exist in the colon.14,19 Mucosal afferents do not respond to distention or contraction but are exquisitely sensitive to mechanical deformation of the mucosa, as might occur with particulate material within the lumen.16,23 In the rat duodenum and jejunum, vagal afferent fibers penetrate the circular muscle layer and submucosa to form networks of multiply branching axons within the lamina propria of both crypts and villi.17 Terminal axons are in close contact with, but do not seem to penetrate, the basal lamina and thus are in an ideal position to detect substances including absorbed nutrients and mediators that are released from epithelial cells and other structures within the lamina propria.
Central Connections of Neural Control Elements
The central connections of the spinal and sympathetic supply to the gut are less well described. The spinal sensory neurons enter the spinal cord, where they synapse ipsilaterally on second-order sensory neurons and also provide direct feedback to sympathetic preganglionic motor neurons through axon collaterals. The second-order sensory neurons then ascend the spinal cord either contralaterally or ipsilaterally, after which they terminate in numerous areas,15 including the raphe nuclei and periaqueductal gray matter in the brainstem and the thalamus. The thalamus has extensive ramifications throughout the CNS. The central influence on sympathetic motor output to the small intestine is complex and not well understood, but stress and arousal level play a role. These influences have their output through the brainstem and descending tracts to the sympathetic preganglionic motor neurons in the intermediolateral horn of the spinal cord, which send their axons to the prevertebral ganglia, whereupon they synapse with sympathetic postganglionic adrenergic nerves.13
GASTROINTESTINAL HORMONES
Gastrointestinal hormones are dealt with in detail in Chapter 1, but it is important to emphasize here their vital role in modulation of small intestinal motor and sensory function. Gastrointestinal hormones relevant to small intestinal function can act in either a humoral or paracrine fashion on both enteric neurons and myocytes, and generally they are released in response to the presence (or anticipation) of enteral nutrition. The best known of these hormones include CCK, somatostatin, VIP, glucagon-like peptide-1 (GLP-1), gastric inhibitory peptide (GIP), ghrelin, and motilin. Most of the hormones released in response to the presence of food in the lumen lead to slowing of small intestinal transit, signals of satiety, and increased mixing or segmenting contractions (see later). For a detailed description of these hormones and their effects, the reader is referred to Chapter 1.