Fig. 29.1
The Bianchi longitudinal intestinal lengthening procedure (Reprinted from Bianchi [160], (a) The bowel and its mesentery are divided longitudinally to yield two vascularized halves of the bowel wall. (b) End-to-end anastomosis of the newly formed bowel loops results in a longer but narrower segment of bowel compared to the original loop. © Elsevier 2006)
A more recent addition to the intestinal lengthening armamentarium is the serial transverse enteroplasty (STEP) [135]. The technique involves the partial transection of dilated bowel using a linear cutting stapler, which is applied sequentially from alternating and opposite directions, in transverse fashion (Fig. 29.2). The goal is to produce a zigzag pattern of lengthened bowel with a diameter of approximately 2 cm. In contrast to the Bianchi procedure, STEP can be employed for recurrent bowel dilatation after previous lengthening [133]. Published results with this technique have been promising. A multicenter registry of 21 SBS patients undergoing STEP reported that the percentage of total calories tolerated enterally increased from 31 to 67 % at a median follow-up of 12.6 months [136]. A single-institution experience that included 34 STEP and 43 Bianchi procedures demonstrated a trend toward a higher rate of weaning from parenteral nutrition in patients who underwent STEP (60 % vs. 55 %) [131]. Long-term outcomes after STEP were reported in a single-center series of 12 pediatric patients; while 2 patients subsequently received liver-intestinal transplants and 2 others died of liver failure, 7 of the remaining 8 patients were weaned off parenteral nutrition by 4 years post-STEP [137].
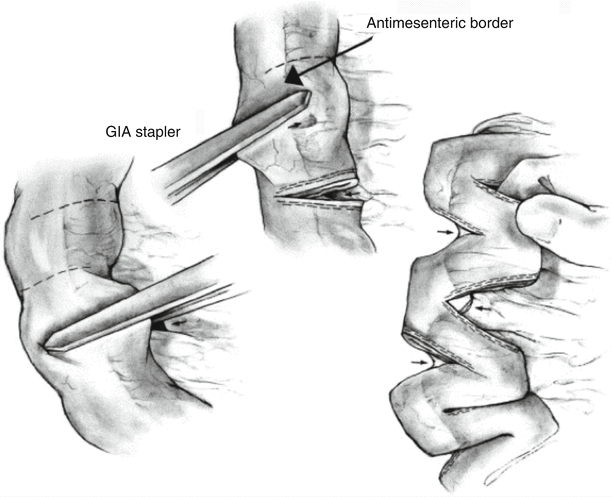

Fig. 29.2
The serial transverse enteroplasty (STEP) procedure (Reprinted from Javid et al. [161] © Elsevier 2005)
Other Non-transplant Procedures
Key Concept: Dilation and adaption of the bowel can be helpful but also can lead to complications that may need to be addressed with other surgical procedures.
As alluded to previously, dilatation of the intestinal remnant normally occurs as an adaptive response following resection in order to slow intestinal transit and increase mucosal absorptive area [124]. However, this compensatory process can lead to pathologic consequences such as dysmotility, bacterial overgrowth, and impairment of absorptive function. For such scenarios in patients with moderately shortened bowel, plication of the bowel wall and tapering enteroplasty may be beneficial [67, 127].
Small Bowel Transplantation
Key Concept: Small bowel transplantation is a viable therapeutic option for intestinal failure as improvements have occurred in immunosuppressive agents. While select patients are typically in the end stage who have failed parenteral nutrition, there is controversy regarding the need to expand this to more patients earlier in their SBS course.
Historically, transplantation of the small intestine was believed to be associated with seemingly insurmountable challenges related to the organ’s immunogenicity and colonization with microorganisms [138]. Earlier efforts were associated with very high rates of morbidity and mortality related to rejection, graft loss, and bacterial translocation leading to sepsis. More recently, refinement of surgical technique in addition to enhanced immunosuppressive and other perioperative strategies has significantly improved outcomes [138, 139]. Therefore, small bowel transplantation has become firmly established as a viable therapeutic option for intestinal failure. Depending on the extent of liver disease and other abdominal pathology, a combined liver-intestine or multivisceral graft may be indicated [139]. Recent data from high-volume intestinal transplant centers demonstrate 1-year patient and graft survival rates that exceed 80 and 70 %, respectively [140–142]. While long-term survival has also dramatically improved in recent decades, they still fall short of outcomes seen with other abdominal organ transplants [143]. The Pittsburgh group reported their series of intestinal and multivisceral transplants divided into time periods; for the 322 transplants performed during the study’s latest era (between 2001 and 2008), 5-year patient and graft survival rates were 68 and 53 %, respectively [142]. There are ongoing efforts to develop novel strategies to overcome late graft loss and its sequelae [142, 144].
Traditionally, intestinal transplantation has been reserved for patients with permanent intestinal failure who can no longer be maintained on total parenteral nutrition therapy [145]. Specifically, patients should be considered for transplantation if they have impending or overt liver failure, repeated loss of central venous access due to thrombosis, recurrent episodes of catheter-related sepsis, or frequent dehydration despite intravenous supplementation [146]. For these patients, prompt referral to a transplant center for evaluation is imperative for optimizing outcome [147, 148]. Early transplantation, as defined by less than 12 months of prior parenteral nutrition therapy, has been shown to be associated with better survival [142]. As clinical outcomes of intestinal transplantation continue to improve, some experts have advocated for the restrictive indications to be broadened [149]. Indeed, there has been increasing debate regarding the role of “preemptive” transplantation in patients who are at high risk of developing parenteral nutrition failure; this may apply to patients with ultrashort small intestine (<50 cm), primary motility disorders, chronic obstruction, and radiation injury [144, 149]. The poor prognosis associated with parenteral nutrition failure supports early consideration of transplantation [149]. Furthermore, there are multiple studies that demonstrate improved quality of life indicators following transplantation [143, 149]. As well, intestinal transplantation has been shown to be cost-effective for managing intestinal failure as long as graft function is maintained for at least 2–3 years after surgery [150].
Future Directions
Key Concept: We remain hampered by a widespread lack of effective options for severe SBS, although emerging technology is in the investigative phase to give patients additional hope.
Despite its recent advances, small intestinal transplantation continues to be limited by issues such as donor availability, graft rejection, and adverse effects related to immunosuppression. As a potential solution to overcome these difficulties, tissue-engineered small intestine has been studied in animal models [151, 152]. The technology makes use of biomaterials such as small intestinal submucosa to generate new tissue and takes advantage of the regenerative ability of intestinal epithelium [153]. While normal structural components have been successfully generated, peristaltic motion of the intestine has yet to be recreated [133]. As well, it may be difficult to procure the necessary neonatal intestinal organelles in humans and to scale up the size of the tissue-engineered intestine to clinically useful dimensions [153]. Nevertheless, if this technology were to become feasible in the future, it has the potential to dramatically alter the management of short bowel syndrome.
Outcomes
Key Concept: The prognosis of patients with short bowel syndrome is determined by their remnant intestinal anatomy and underlying disease and modulated by their response to medical and surgical treatments.
Overall, patients who are dependent on home parenteral nutrition (HPN) have higher mortality than their age-matched counterparts in the general population [154]. A French group recently reported their results over a 25-year period including 268 consecutive adult SBS patients who required HPN [66]. Survival was 94, 70, and 52 % at 1, 5, and 10 years, respectively. Complications related to SBS and HPN combined accounted for only 26 % of the mortality. The study also found the probabilities of a patient remaining dependent on HPN were 74, 64, and 48 % at 1, 2, and 5 years, respectively. Factors significantly associated with HPN dependence at 5 years included remnant small intestinal length of less than 75 cm, less than 4/7 of colon remaining, and postoperative citrulline concentration of less than 20 μmol/L. Comparable results have been reported by other centers regarding the prognosis of HPN-dependent patients, including 5-year survival rates ranging between 60 and 78 % [65, 155, 156].
There have been few studies addressing quality of life (QOL) of patients on HPN. Jeppesen et al. used two validated (QOL) questionnaires on 49 HPN-dependent patients and 36 patients who did not receive HPN but had anatomical or functional short bowel [157]. Compared to the latter group, the former was found to have a poorer quality of life that was comparable to that reported for dialysis-dependent patients with chronic renal failure. Another research demonstrated that lowest QOL scores are more common during the first year on HPN, particularly if the patient was previously well [158]. Quality of life then gradually improves under its plateaus after 4–5 years on HPN. A US study found low quality of life in patients requiring long-term HPN to be associated with length of time on total parenteral nutrition, lack of family supports, and financial difficulties [159]. With respect to intestinal transplantation, there is increasing evidence that it results in improved quality of life measures [144]. In a comparison of QOL measures between 79 adult transplant survivors and 79 HPN patients, Abu-Elmagd et al. reported superior results with transplantation across several psychological, emotional, and social domains [143].
Summary Pearls
You will be confronted with patients with SBS, and they may be some of the most challenging that you will encounter. It is important to remember that the management of the patient with short bowel syndrome is guided by a thorough understanding of the remnant intestinal anatomy and physiology as well as the underlying disease (Fig. 29.3). These factors will largely determine the patient’s clinical manifestation, which may range from mild malabsorption correctable with dietary modifications to intestinal failure requiring complex bowel rehabilitation and surgical strategies. As outcomes in published series consistently correlate with residual length of small intestine, it is worthwhile during the initial resection operation to preserve as much of it as possible. Similarly, an intact colon is valuable as it can compensate for the lost absorptive function, and its presence is associated with independence from home parenteral nutrition.
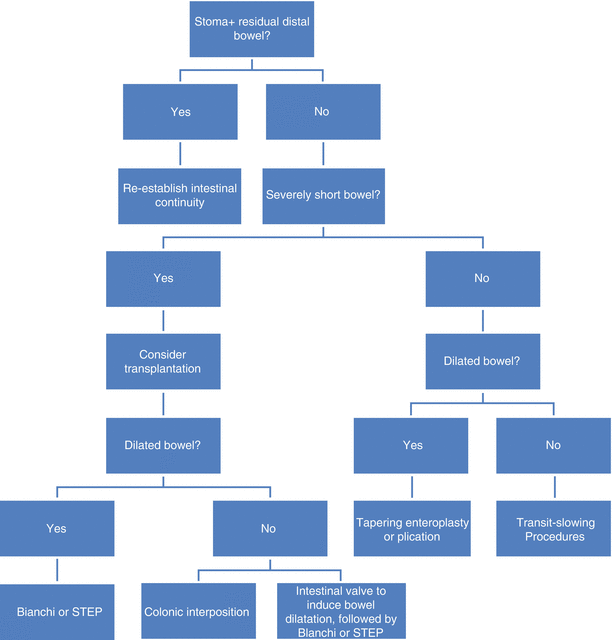

Fig. 29.3
Algorithm for surgical management of short bowel syndrome
A multidisciplinary approach is essential for the optimal care of these complex patients. In particular, individuals who are dependent on home parenteral nutrition should be managed by a center with appropriate expertise and resources. This is likely to optimize intestinal rehabilitation, reduce complications associated with long-term parenteral nutrition, and facilitate access to specialized medical and surgical therapies. For nutritional support, you should use the enteral route whenever possible; the presence of luminal nutrients is necessary for intestinal adaption, a process which may continue for several years following resection. Antisecretory and antimotility medications may be useful adjuncts for reducing water and salt losses. Among the growth factors, GLP-2 and its analogue, teduglutide, are promising intestinotrophic agents that can augment a bowel rehabilitation regimen.
In the absence of contraindications to surgery, you should attempt to restore intestinal continuity. For patients whose bowel is already in continuity, but cannot wean off parenteral nutrition despite seemingly adequate intestinal length, segmental reversal of small bowel should be considered to slow transit. On the other hand, for patients who are clearly limited by a very short bowel that is dilated, either the Bianchi procedure or STEP may be appropriate. Of these two bowel-lengthening operations, STEP is likely easier to perform and can be used as a repeat procedure. Finally, intestinal transplantation has evolved over recent years to offer improved survival and quality of life outcomes. Most importantly, for SBS patients with adverse risk factors for failing parenteral nutrition or if you do not feel comfortable or have the resources to care for these patients, referral for evaluation regarding transplantation should be considered early in their course.
References
1.
Buchman A, Scolapio J, Fryer J. AGA technical review on short bowel syndrome and intestinal transplantation. Gastroenterology. 2003;124:1111–34.PubMed
2.
Jeejeebhoy KN. Management of short bowel syndrome: avoidance of total parenteral nutrition. Gastroenterology. 2006;130(2 Suppl 1):S60–6.PubMed
3.
Buchman AL. Etiology and initial management of short bowel syndrome. Gastroenterology. 2006;130(2 Suppl 1):S5–15.PubMed
5.
Nightingale J, Woodward JM. Guidelines for management of patients with a short bowel. Gut. 2006;55 Suppl 4:iv1–12.PubMedCentralPubMed
6.
Longshore S, Wakeman D, McMellen M, Warner B. Bowel resection induced intestinal adaptation: progress from bench to bedside. Minerva Pediatr. 2009;61(3):239–51.PubMed
7.
Jeejeebhoy KN. Short bowel syndrome: a nutritional and medical approach. CMAJ. 2002;166(10):1297–302.PubMedCentralPubMed
8.
Thompson W, Wrathell E. The relation between ileal resection and vitamin B12 absorption. Can J Surg. 1977;20(5):461.PubMed
9.
Hofmann AF. Bile acid malabsorption caused by ileal resection. Arch Intern Med. 1972;130(4):597–605.PubMed
10.
Lin H, Zhao X, Wang L. Intestinal transit is more potently inhibited by fat in the distal (ileal brake) than in the proximal (jejunal brake) gut. Dig Dis Sci. 1997;42(1):19–25.PubMed
11.
Stanley S, Wynne K, Bloom S. Gastrointestinal satiety signals III. Glucagon-like peptide 1, oxyntomodulin, peptide YY, and pancreatic polypeptide. Am J Physiol Gastrointest Liver Physiol. 2004;286:G693–7.PubMed
12.
Nightingale J, Kamm M, van der Sijp J, Ghatei M, Bloom S, Lennard-Jones J. Gastrointestinal hormones in short bowel syndrome. Peptide YY may be the “colonic brake” to gastric emptying. Gut. 1996;39:267–72.PubMedCentralPubMed
13.
Fordtran J, Rector F, Carter N. The mechanisms of sodium absorption in the human small intestine. J Clin Invest. 1968;47(4):884–900.PubMedCentralPubMed
14.
Davis G, Santa Ana C, Morawski S, Fordtran J. Permeability characteristics of human jejunum, ileum, proximal colon and distal colon: results of potential difference measurements and unidirectional fluxes. Gastroenterology. 1982;83(4):844–50.PubMed
15.
Chaet M, Farrell M, Ziegler M, Warner B. Intensive nutritional support and remedial surgical intervention for extreme short bowel syndrome. J Pediatr Gastroenterol Nutr. 1994;19(3):295–8.PubMed
16.
Sondheimer J, Cadnapaphornchai M, Sontag M, Zerbe G. Predicting the duration of dependence on parenteral nutrition after neonatal intestinal resection. J Pediatr. 1998;132:80–4.PubMed
17.
Andorsky D, Lund D, Lillehei C, Jaksic T, DiCanzio J, Richardson D, et al. Nutritional and other postoperative management of neonates with short bowel syndrome correlates with clinical outcomes. J Pediatr. 2001;139:27–33.PubMed
18.
Dibaise JK, Young RJ, Vanderhoof JA. Enteric microbial flora, bacterial overgrowth, and short-bowel syndrome. Clin Gastroenterol Hepatology. 2006;4(1):11–20.
19.
Vanderhoof J, Young R, Murray N, Kaufman S. Treatment strategies for small bowel bacterial overgrowth in short bowel syndrome. J Pediatr Gastroenterol Nutr. 1998;27(2):155–60.PubMed
20.
Kaufman S, Loseke C, Lupo J, Young R, Murray N, Pinch L, et al. Influence of bacterial overgrowth and intestinal inflammation on duration of parenteral nutrition in children with short bowel syndrome. J Pediatr. 1997;131:356–61.PubMed
21.
Fich A, Steadman C, Phillips S, Camilleri M, Brown M, Haddad A, et al. Ileocolonic transit does not change after right hemicolectomy. Gastroenterology. 1992;103(3):794–9.PubMed
22.
Fry R, Mahmoud N, Maron D, Ross H, Rombeau J. Colon and rectum. In: Townsend C, Beauchamp R, Evers B, Mattox K, editors. Sabiston textbook of surgery. 18th ed. Philadelphia: Saunders, Elsevier; 2007.
23.
Joly F, Mayeur C, Messing B, Lavergne-Slove A, Cazals-Hatem D, Noordine M-L, et al. Morphological adaptation with preserved proliferation/transporter content in the colon of patients with short bowel syndrome. Am J Physiol Gastrointest Liver Physiol. 2009;297(1):G116–23.PubMed
24.
Royall D, Wolever T, Jeejeebhoy K. Evidence for colonic conservation of malabsorbed carbohydrate in short bowel syndrome. Am J Gastroenterol. 1992;87(6):751–6.PubMed
25.
Gouttebel MC, Saint-Aubert B, Astre C, Joyeux H. Total parenteral nutrition needs in different types of short bowel syndrome. Dig Dis Sci. 1986;31(7):718–23.PubMed
26.
Nightingale JM, Lennard-Jones JE, Gertner DJ, Wood SR, Bartram CI. Colonic preservation reduces need for parenteral therapy, increases incidence of renal stones, but does not change high prevalence of gall stones in patients with a short bowel. Gut. 1992;33(11):1493–7.PubMedCentralPubMed
27.
Mitchell JE, Breuer RI, Zuckerman L, Berlin J, Schilli R, Dunn JK. The colon influences ileal resection diarrhea. Dig Dis Sci. 1980;25(1):33–41.PubMed
28.
Thompson J. Short bowel syndrome and Crohn’s disease. J Gastrointest Surg. 2003;7(8):1069–72.PubMed
29.
Polito J, Childs B, Mellits E, Tokayer A, Harris M, Bayless T. Crohn’s disease: influence of age at diagnosis and site and clinical type of disease. Gastroenterology. 1996;111(3):580–6.PubMed
30.
Agwunobi A, Carlson G, Anderson I, Irving M, Scott N. Mechanisms of intestinal failure in Crohn’s disease. Dis Colon Rectum. 2001;44(12):1834–7.PubMed
32.
Kotanagi H, Kramer K, Fazio VW, Petras RE. Do microscopic abnormalities at resection margins correlate with increased anastomotic recurrence in Crohn’s disease? Retrospective analysis of 100 cases. Dis Colon Rectum. 1991;34(10):909–16.PubMed
33.
Yamamoto T, Fazio VW, Tekkis PP. Safety and efficacy of strictureplasty for Crohn’s disease: a systematic review and meta-analysis. Dis Colon Rectum. 2007;50(11):1968–86.PubMed
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree








