Fig. 1
Patient is placed in a supine position with a towel placed under the scrotum
Fig. 2
Patient positioning: the phallus is positioned up on the pubis, held by the patient or a towel
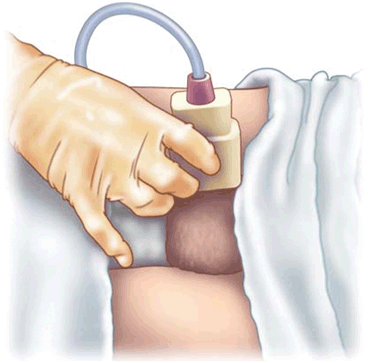
Fig. 3
Sonographer performing a longitudinal view of the testis. Note the use of the fifth finger on the patient’s thigh to help steady the transducer and minimize movement of the testis in the scrotum
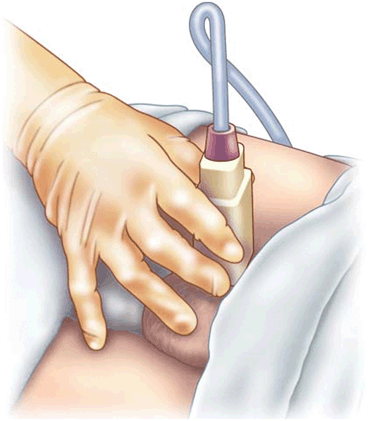
Fig. 4
Sonographer positioning the transducer for a transverse view of the testis. Note again, the use of the fifth finger on the patient’s thigh to help steady the transducer and minimize movement of the testis in the scrotum
Transducer Selection
The choice of the frequency used is determined by a balance between depth of penetration required and the detail of the image required. As the frequency increases the image resolution (axial resolution) improves and the depth of penetration decreases. Broad bandwidth transducers allow for multiple focal zones, eliminating the need for adjustment during the examination. Multiple frequency transducers allow the transducer to be set to several distinct frequencies. A high frequency (7.5–18 MHz) array transducer is most often used for scrotal scanning. A linear array probe with a “footprint” able to measure the longitudinal length of testis is ideal. A curved array probe can be used with a thickened scrotal wall or in the presence of scrotal edema or for a large testis. The curved array transducer is also useful to compare echogenicity of the testes, however, the frequency is usually lower with a curved array probe, resulting in decreased axial resolution. Color and spectral Doppler are essential elements of scrotal ultrasound because they provide documentation of testicular blood flow and paratesticular findings. The highest possible frequency, normally in the 7.5–18 MHz range, providing the best axial resolution and blood flow detection, should be used [1].
Overview of the Examination
In the longitudinal view, the standard orientation of the image should be with the superior pole of the testis to the left and the inferior pole to the right on the monitor screen (Fig. 5). In the transverse plane, the standard orientation is for the patient’s right testis to be on the right side of the screen. Therefore, for the right testis, the lateral aspect is located on the left side of the screen and the medial aspect to the right. Conversely, for the left testis, the lateral aspect should be to the right and the medial aspect to the left (Fig. 6).
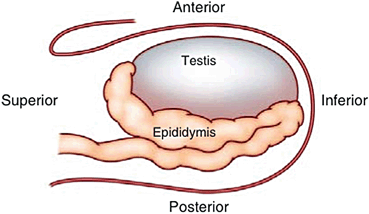
Fig. 5
Schematic view of longitudinal scrotal ultrasound
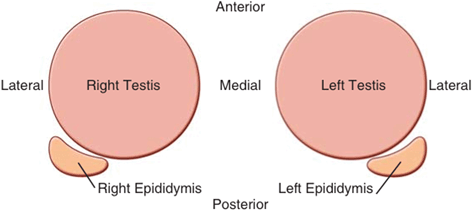
Fig. 6
Schematic view of transverse scrotal ultrasound as seen on the ultrasound screen with the right testis on the left and left testis on the right. The relative positions of each epididymis are also demonstrated
The evaluation of the scrotal contents should begin with a longitudinal survey scan, progressing medial to lateral to get an overall impression of the testis and paratesticular structures . If the testis is larger than the footprint of the transducer, it is important to document views of the superior and inferior portions of the testis including the epididymis in these regions. The transverse view is obtained by rotating the transducer to 90°. A survey scan is performed using the mid-testis as a starting point and proceeding first towards the superior pole then back to the to mid-testis before scanning to the inferior pole.
At least one image should visualize both testes to document the presence of two testes and their relative echogenicity (Fig. 7). Measurements of the testicular width and height are taken and documented at the mid-testis. A measurement should also be made of the long axis at the mid-testis and a testicular volume is calculated (Fig. 8). If the equipment being used has split-screen capabilities, comparative views of echogenicity can easily be made and documented.
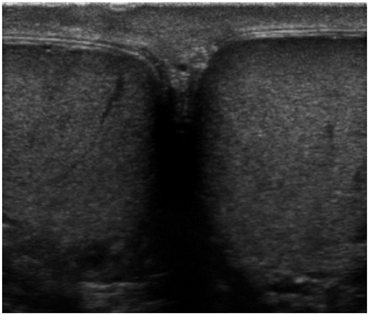
Fig. 7
Gray scale side-by-side view of both testes in a single image. This image is important to confirm the presence of two testes
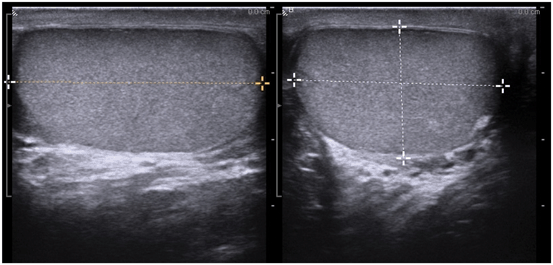
Fig. 8
Gray scale ultrasound in transverse and longitudinal planes used to measure the testicular volume
All relevant extratesticular structures should be evaluated, including but not limited to the epididymis , spermatic cord, and scrotal skin. Techniques that improve visualization, such as Valsalva maneuver or upright positioning, may be used as needed.
Color and Spectral Doppler
Color and spectral Doppler should be considered an integral part of the scrotal US examination. Many inflammatory, neoplastic, and benign conditions have characteristic flow patterns that can assist in diagnosis. At least one side-by-side image containing both testes with identical Doppler settings should be included to evaluate symmetry of flow. If blood flow cannot be visualized on color Doppler (Fig. 9a), power Doppler may increase the sensitivity to detect blood flow (Fig. 9b) [1].
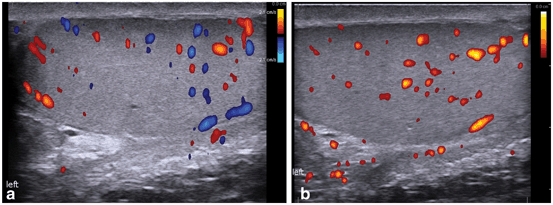
Fig. 9
a Color Doppler ultrasound demonstrating testicular blood flow. b Power Doppler ultrasound demonstrating testicular blood flow. Note lack of directionality with power Doppler
Documentation
The written report and archived images are a reflection of the quality of the examination. The old adage “If it’s not documented, it wasn’t done” should guide the sonographer in developing a quality report. The static images obtained during the evolving ultrasound examination should represent the sonographer’s impression of the findings. If electronic storage space is available and the equipment allows, video clips, which demonstrate important findings and survey scans, can and should be obtained. A quality report can aid in diagnosis, and is therefore in the best interest of our patients.
All the measurements and anatomical findings of the examination should be documented. Images should be attached to the report. It is essential to include patient identification information, the examination date, and the indications for performing the examination. The transducer used and its frequency should also be documented. The area of interest should be clearly identified. The orientation and measurements should be labeled along with the pertinent anatomy and any abnormalities. There is no minimum number of images that are required for proper documentation. It is a best practice to provide images that depict the measurements being taken and the pathology being described. The physician who performed the examination should sign the report.
Indications
There are many specific indications for scrotal ultrasound (Table 1). Scrotal ultrasound is often performed when the physical examination is inconclusive or difficult to complete (or both) because of patient discomfort or inability of the examiner to precisely identify the scrotal structures on palpation. In these instances the scrotal ultrasound examination is therefore an integral part of the physical examination of the male genitalia. In other situations ultrasound evaluation is essential to diagnosis and treatment and is well supported in the literature. However, the decision on whether or not to obtain an ultrasound study is discretionary and without a clearly defined evidence based approach. “Appropriateness” criteria are necessary and high level evidence base studies are required in order to determine appropriateness. Part of the work of urologic imaging research in the future should look to assess the limitations of the current literature and then create an evidence base that will define the value of the imaging services critical to the practice of urology [2, 3].
Table 1
Indications for scrotal ultrasound
Assessment of scrotal mass |
Painful enlargement |
Epididymitis/orchitis |
Testicular abscess |
Torsion |
Non-painful enlargement |
Testicular tumor |
Hydrocele |
Varicocele |
Spermatocle/epididymal cyst |
Scrotal hernia |
Cyst |
Evaluation of scrotal trauma |
Testicular rupture |
Hematocele |
Evaluation and management |
Detection of occult primary tumors in patients with metastatic germ cell tumors |
Follow-up of patients with prior primary testicular neoplasms, leukemia, or lymphoma |
Evaluation of abnormalities noted on other imaging modalities (CT., MRI, PET, etc.) |
Evaluation of intersex conditions |
Investigation of empty/abnormal scrotal sac |
Undescended testis |
Thickened scrotal skin |
Evaluation of male infertility and related issues |
Varicocele |
Intratesticular microcirculation |
Atrophic testis |
Microlithiasis |
Impaired semen quality |
Azoospermia |
Antisperm antibody |
Post operative follow up |
Varicocele |
Testis biopsy |
Hydrocelectomy |
Patients with indeterminate scrotal masses |
Normal Anatomy of the Testis and Paratesticular Structures
The normal scrotal wall thickness varies between 2 and 8 mm. The scrotal wall contains the following structures: rugated skin, superficial fascia, dartos muscle, external spermatic fascia, cremasteric fascia and internal spermatic fascia . The scrotum is separated into right and left hemiscrotal compartments by a septum termed the median raphe. As the testis descends in utereo from the abdomen, it acquires each layer of the scrotal compartment. The external spermatic fascia is derived from the external oblique fascia and is attached to the external inguinal ring. The cremasteric fascia and muscle derive from the internal oblique muscle. Encasing each testis is the tunica vaginalis, derived from the peritoneum, which consists of parietal and visceral layers. These layers are normally separated by 2–3 ml of straw colored fluid often referred to as a physiologic hydrocele. Ultrasound of this fluid is seen as a thin echo free rim around the head of the epididymis [4] (Fig. 10). The parietal and visceral layers join at the posterolateral aspect of the testes where the tunica attaches to the scrotal wall [5] .
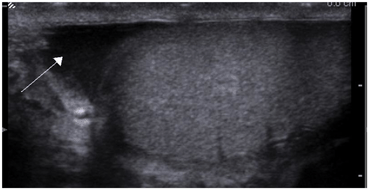
Fig. 10
Gray scale ultrasound showing physiologic hydrocele (arrow)
The testes descend into the scrotum at approximately the 28th week of gestational age through the inguinal canal along with the processus vaginalis. The processus vaginalis gradually closes through infancy and early childhood. The size and shape of the testis changes with the age, influenced by gonadotropic hormones testicular volume gradually rises from birth to up to 5 months of age due to peak in gonadotropic hormones levels [6, 7]. After 5 months of age, the testicular volume steadily declines and reaches its minimum volume at approximately 9 months of age and remains approximately the same size until puberty [8]. In newborns, the testis is round and gradually becomes ovoid with growth. The echogenicity of the testis increases in puberty due to the development of germ cell elements [9] .
The adult testis is a smooth ovoid gland, approximately 4–5 cm long, 3 cm wide, 2–3 cm in the anterior-posterior (AP) dimension, and typically between 20 and 30 ml in volume. The testis exhibits medium homogenous echogenicity. A dense fibrous capsule the tunica albuginea envelops the testis, which is apparent as a thin echogenic line on ultrasound. Each testis has approximately 200–300 cone shaped lobules each containing at least one seminiferous tubule [10] (Fig. 11). The lobules are separated by the fibrous septa of tunica albuginea that extend from the mediastinum of the testis in to the parenchyma of the testis [11]. Testicular lobules are occasionally identified on ultrasound as lines radiating from the mediastinum testis (Fig. 12). The seminiferous tubules contained within the lobules open into dilated spaces called rete testis within the mediastinum. The seminiferous tubules are long V-shaped tubules, both ends of which usually terminate in the rete testis. The rete testis is connected to the head (caput or globus major) of the epididymis with about 8–12 efferent ductules. The normal rete testis is sonographically evident in 18 % of patients as a hypoechoic area with a striated configuration adjacent to the mediastinum testes [12] . The mediastinum testes appears as a linear avascular echogenic band on ultrasonography [13] (Fig. 13).
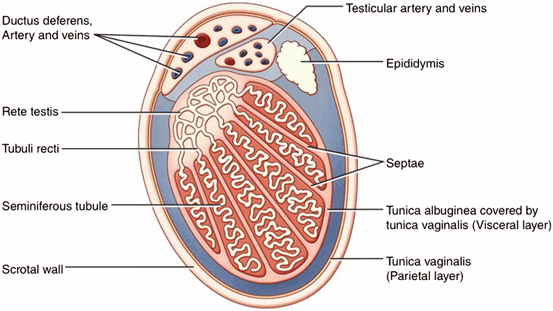
Fig. 11
Schematic cross section of the testis
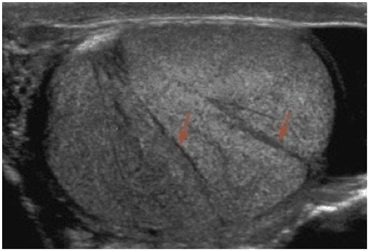
Fig. 12
Gray scale ultrasound of a normal testis demonstrating testicular lobules separated by fibrous septa (arrows)
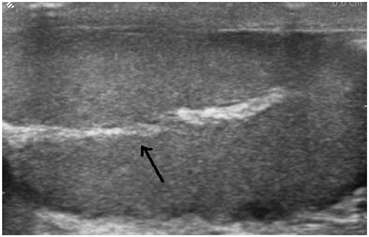
Fig. 13
The mediastinum testis appears as an avascular echogenic line (arrow)
The adult epididymis is 6–7 cm long and has three parts, the head (caput) measuring 10–12 mm in diameter, the body (corpus) measuring 2–4 mm in diameter, and the tail (cauda) about 2–5 mm in diameter. In the normal epididymis, the head is routinely identified at posterolateral to the upper pole of the testis. The caput epididymis is triangular in shape, often has the same echogenicity as the testis (Fig. 14). However, it can be heterogenous with areas that are hyper- or hypoechogenic. The smaller corpus epididymis can be seen as a hypoechoic structure containing multiple echogenic linear structures representing the coiled epididymal tubule, and lies posteriorly along the long axis of the testis (Fig. 15).
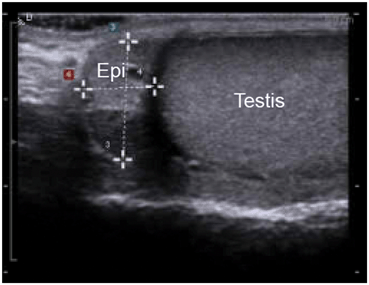
Fig. 14
The caput epididymis is triangular in shape (E) and is usually isoechoic or slightly hypoechoic compared to the testis
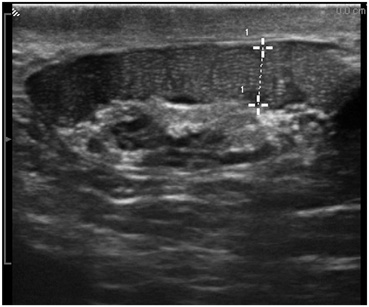
Fig. 15
Gray scale image of a dilated corpus epididymis in a vasectomized man
The testicular appendages are the remnants of the mesonephric and paramesonephric ducts. There are four testicular appendages: the appendix testis, the appendix epididymis, the vas aberrans, and the paradidymis [14] (Fig. 16). The appendix testis and the appendix epididymis are commonly seen on scrotal ultrasound [15]. The appendix testis (hydatid of Morgagni) is a small ovoid structure usually at the upper pole of the testis in the groove between the testis and the epididymis, better seen in the presence of fluid around the testis. The appendix testis is the vestigial remnant of the paramesonephric (Mullerian) duct (Fig. 17). The appendix epididymis originates from the mesonephric (Wolffian) duct and is seen associated with the epididymal head on ultrasound images (Fig. 18) .
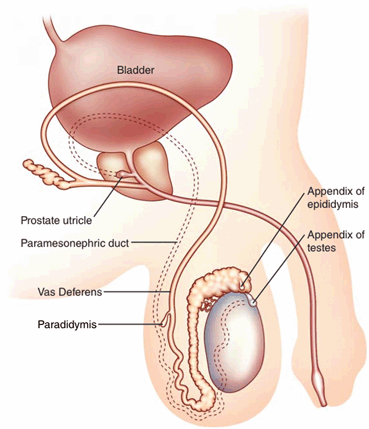
Fig. 16
Schematic showing the most frequent location of testicular and epididymal appendages
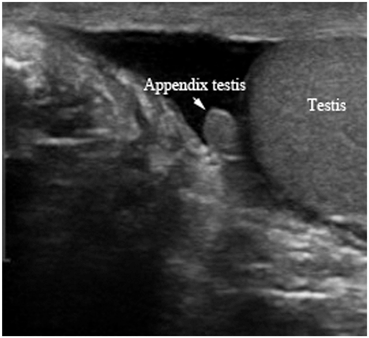
Fig. 17
B mode (also called Gray scale) ultrasound showing the appendix testis
Fig. 18
Gray scale ultrasound showing the appendix epididymis
The spermatic cord is normally seen superior to the posteromedial aspect of the testis and contains the vas deferens, the testicular and cremasteric and deferential arteries, the pampiniform plexus of veins, genital branch of the genital femoral nerve, testicular plexus of the sympathetic trunk, and lymphatic vessels [11]. The blood supply to the scrotal structures is from three primary arteries: the testicular, deferential, and cremasteric arteries (Fig. 19). The testicular artery testis or gonadal artery, which arises from the aorta and courses through the scrotum with the spermatic cord, is the major supply to the testis. The deferential artery, which arises from the superior vesical artery and supplies the vas deferens and epididymis . The cremasteric artery, a branch of the inferior epigastric artery, which supplies the scrotal skin and coverings of the spermatic cord. As the testicular artery approaches the posterolateral aspect of the testis it divides. The branches pierce through the tunica albuginea to run in a layer called the tunica vasculosa. Capsular arteries run peripherally in the tunica vasculosa, supplying centripetal arteries that course towards the mediastinum and divide further to recurrent rami that flow away from the mediastinum [13]. The veins draining the testis and epididymis converge to form the pampiniform plexus at the mediastinum on the superior pole of the testis. The pampiniform plexus is primarily drained by the testicular and external pudendal veins [16]. The testicular vein on the left drains into the renal vein and the testicular vein on the right directly into the inferior vena cava [17].
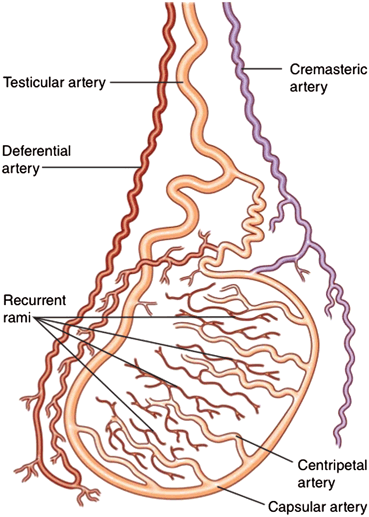
Fig. 19
Blood supply of testis. The testis is supplied by three sources of blood supply. 1. The testicular artery, which arises from the aorta. 2. The deferential artery, which arises from the superior vesical artery. 3. The cremasteric artery, a branch of the inferior epigastric artery
Ultrasongraphy with Color-Flow imaging provides visualization of the intratesticular, epididymal, and paratesicular blood flow. Under normal conditions, Color Flow images show equivalent vascularity of the bilateral testis. When vascularity is not well visualized, Power Doppler increases the sensitivity of detection. Spectral Doppler is used to calculate the Resistive Index (RI) of intratesticular arteries. The RI of intratesticular arteries has been found to correlate with testicular function, namely spermatogenesis .
Sonoelastography
The ability to access pathology by palpation has long been a key part of the physician’s physical examination . Hard lesions are often a sign of pathology. Sonoelastographraphy (tissue elasticity imaging) is an evolving ultrasound modality which adds the ability to evaluate the elasticity of biological tissues. Essentially, it gives a representation, using color, of the softness or hardness of the tissue of interest. The physics of this modality is given in an earlier chapter. Visually, the elasticity of a tissue is represented by color spectrum. Be aware that the color given to hard lesions is determined by the manufacturer of the equipment as well as being able to be set by the user. Therefore, just as in using color Doppler, the user needs to look at the color bar to know what color represents a “hard” and “soft” lesion .
Testicular Pathology
Malignant Lesions of the Testis
Testicular malignancies account for approximately 1 % of all the malignancies in men . The predicted 5-year survival rate is approximately 95 %, believed to be due to early detection as patient appreciation of abnormality in a superficially palpable organ and tumor sensitivity to chemotherapy and radiotherapy. The most common presentation is of a painless scrotal mass, with pain being reported in only 10 % of cases [18]. Ultrasonography is the gold standard imaging modality for diagnosis. Ultrasound characteristics differ significantly for malignant as compared to benign (Table 2) intratesticular masses .
Table 2
Ultrasound characteristics of non-neoplastic intratesticular masses
Intratesticular lesion | Ultrasound findings |
|---|---|
Simple testicular cyst | Well-circumscribed, anechoic, increased through-transmission |
Epidermoid cyst | Classic appearance: an onion ring due to alternating layers of hypoechogenicity and hyperechogenicity |
Tubular ectasia of the rete testis (TERT) | Avascular cystic dilations in the rete testis |
Intratesticular varicocele | Anechoic, tortuous structure with a venous waveform on Doppler color flow study |
Tunica albuginea cyst | Cyst at upper or lateral margin of testis; may be clinically palpable |
Testicular hematoma | Avascular hyperechoic lesion within the testicular parenchyma |
Congenital testicular adrenal rests | Bilateral hypoechoic or hyperechoic lesions with or without posterior acoustic shadowing |
Germ Cell Tumors
Germ cell tumors account for 95 % of testicular malignancies and can be divided into seminomatous and non-seminomatous germ cell tumors. The remaining minority of testis tumors are histologically sex cord stromal tumors, lymphomas, or metastases .
The most common germ cell tumor is seminoma, which comprises up to 50 % of all germ cell tumors. Seminoma occurs in men predominantly between the ages of 35 and 45. Bilateral seminomatous germ cell tumors are rare and are reported only 2 % of the cases [19]. On gross pathology, they are lobulated and pale in color and may vary in size from small well-defined lesions to masses that completely replace normal testicular parenchyma [19]. The sonographic appearance of seminoma typically is a homogeneous well-defined hypoechoic lesion, with cystic areas found only in 10 % of cases [20]. Larger tumors tend to be more heterogeneous and may have poorly defined margins, are often diffusely infiltrative and multifocal (Fig. 20a, 20b).
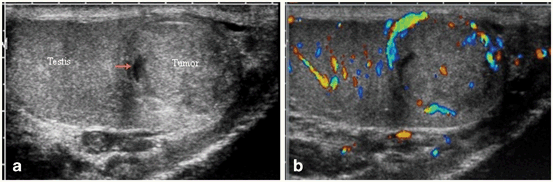
Fig. 20
a Testicular germ cell tumor showing heterogenous appearance on gray scale ultrasound. b Color Doppler flow showing increased blood flow within the tumor
Nonseminomatous germ cell tumors (NSGCT) generally occur in younger men between ages 25 and 35. NSGCT are mixed germ cell tumors comprised of embryonal carcinoma, yolksac tumor, choriocarcinoma and teratoma . They can be locally aggressive with invasion of the tunica albuginea or the epididymis or the spermatic cord. The ultrasonographic findings reflect the diversity of the components and characteristically appear irregular with a heterogeneous parenchyma pattern, representing calcification, hemorrhage, or fibrosis and cystic lesions [21, 22] (Fig. 21a, 21b).
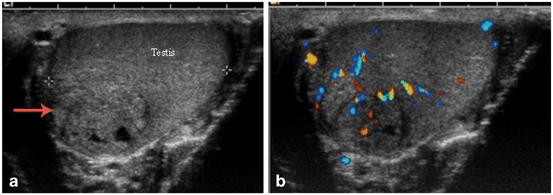
Fig. 21
a Non-seminomatous germ cell tumor showing heterogeneous appearance on gray scale ultrasound. b Color Doppler flow study demonstrating increased blood flow within the tumor
Pure embryonal cell carcinoma makes up 2–3 % of all germ cell tumors. An embryonal cell carcinoma is an aggressive tumor with ultrasonographic findings often demonstrating irregular and indistinct margins, with a heterogeneous echotexture. These tumors are characteristically smaller in size without enlargement of the testis [23]. Yolk sac tumor, also known as endodermal sinus tumor or infantile embryonal carcinoma, most commonly occurs in children younger than 2 years [23]. The sonographic appearance of yolk sac tumors of the testis is inhomogeneous and can have areas of hemorrhage, however it is difficult to differentiate from other solid tumors of the testes based solely on ultrasonography [24]. Choriocarcinoma carries the worst prognosis of all germ cell tumors, with early metastatic spread to the lung, liver, gastrointestinal tract, and brain, and is associated with an elevated human chorionic gonadotropin level. Ultrasound is characterized by cystic and solid areas, corresponding to areas of hemorrhagic necrosis [24] .
Teratoma is the second most common pediatric testicular tumor, and a mature teratoma is often benign in children. A teratoma will demonstrate endodermal, mesodermal and ectodermal components in a disorganized arrangement [21]. Echogenic foci in these tumors represent elements of its embryologic composition—immature bone, fat, and fibrosis.
Epidermoid cysts of the testis are rare benign germ cell tumors. Epidermoid cysts usually present between 20 and 40 years of age. They are normally unilateral, however bilateral occurrence is rarely reported [25]. Epidermoid cysts are variable on sonographic appearance attributable to their contents with keratin and variable maturation. The characteristic “onion ring” sonographic appearance of the epidermoid cyst is due to alternating layers of hypo- and hyperechogenicity without internal flows [15, 26, 27] (Fig. 22a, 22b). An epidermoid cyst of less than 3 cm in size with negative tumor markers can be managed conservatively by enucleation provided that frozen sections are obtained to confirm the diagnosis [28].
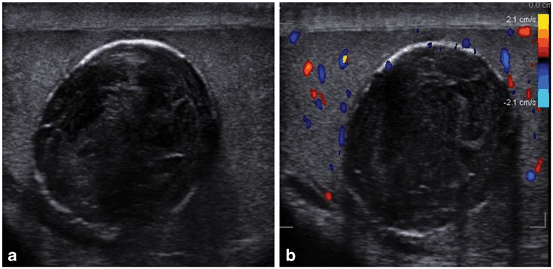
Fig. 22
a The Gray scale appearance of an epidermoid cyst with classic onion ring configuration. b Color Doppler flow study shows no blood flow
Non-germ Cell Tumors
Non-germ cell tumors are rare, but most commonly arise from Leydig or Sertoli cells. Leydig cells are the principle source of male testosterone . Leydig cell tumors represent fewer than 3 % of all testis tumors, they are usually benign but have malignant potential. Male patients have virilizing or feminizing characteristics due to androgen secretion. Ultrasound features are nonspecific, but if Leydig cell tumor is suspected, tumor enucleation may be performed. Sertoli cell tumors represent approximately 1 % of testicular tumors and can occur in children and adults. Sertolic cell tumors are usually found in patients younger than 40, do not secrete horomones, and can occur bilaterally in 20 % of patients [29]. Both Leydig cell and Sertoli cell tumors may be amenable to testis-sparing resection, where intra-operative ultrasonography is an essential component of the procedure .
Testicular Lymphoma
Primary testicular lymphoma is the most common testicular malignancy in men over the age of 60 [30–32]. The most common histological type is large B-cell non-Hodgkins lymphoma. The ultrasound demonstrates diffuse enlargement of the testis with increased vascularity on Doppler color flow (Fig. 23a, 23b). Orchidectomy had been advocated as diagnostic and therapeutic procedure. The treatment recommendation was recently changed to a combined modality of systemic doxorubicin-based chemotherapy, prophylactic intrathecal chemotherapy and orchidectomy or scrotal radiotherapy [31]. Lymphoma found in the testis may also be the initial site found with widespread disease or the site of recurrence for previously treated lymphoma as the testis a sanctuary organ due to the blood-gonad barrier that blocks accumulation of chemotherapy agents [24].
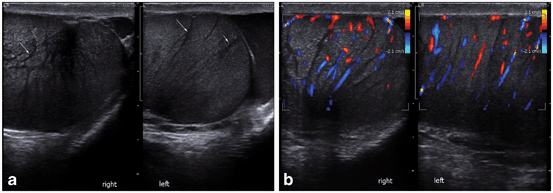
Fig. 23
a Gray scale ultrasound showing bilateral testicular lymphoma. Arrows showing dilated vascular markings. b Doppler color flow study showing increased vascularity in both the testes
Metastasis
Regressed or Burn-Out Germ Cell Tumor
Some patients present with widespread metastatic disease, to the retroperitoneum or beyond, without identification of a primary tumor. Scrotal ultrasound performed for these patients may find an area of calcification in the testis, representing the “burnt-out” primary lesion. One theory to explain the genesis of the burned out tumor is that the tumor outgrows its blood supply and then subsequently involutes, resulting in fibrosis and calcification [35] (Fig. 24).
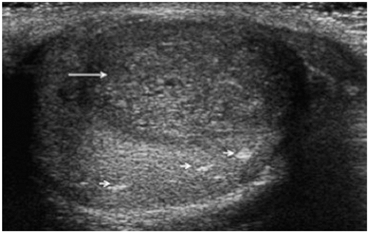
Fig. 24
Gray scale ultrasound showing a mixed germ cell tumor (arrow) and areas of burnt-out tumor (small arrows)
Incidentally Discovered Non-palpable Testicular Lesions
Incidentally found solid testicular masses that are not palpable are usually benign. Significant risk factors for the presence of malignancy include size greater than 1 cm, ipsilateral atrophy, history of cryptorchidism, history of contralateral germ cell tumor, and severe oligospermia or azoospermia [36]. Previous work has shown that patients at low risk for malignancy can be managed with active ultrasound surveillance, proceeding with testis sparing excision biopsy or radical orchiectomy if the lesion size should increase in size [37, 38]. Patients at high risk for malignancy were managed with an ultrasound guided testis sparing excisional biopsy or radical orchiectomy [39].
With the introduction of sonoelastography , non-palpable lesions can be differentiated into “hard” or “soft”. Two recent studies have used real-time elastography to attempt to separate benign from malignant testicular lesions, as it is postulated that malignant lesions have an increased stiffness due to a higher concentration of vessels and cells compared to surrounding tissues (Fig. 37a–37c). Goddi et al. assessed 88 testis with 144 lesions and found a 93 % positive predictive value, 96 % negative predictive value, and 96 % accuracy in differentiating benign from malignant lesions [40]. Similarly, Algner et al. assessed 50 lesions and found a 92 % positive predictive value, 100 % negative predictive value, and 94 % accuracy [41]. Real time tissue elastography is an exciting new innovation in assessing abnormalities on scrotal examination, and may be used to predict the risk of malignancy in a testicular lesion. We believe that this modality may be used to avoid surgical intervention on benign lesions based on the finding the lesion to be “soft” on elastography. A possible approach to the evaluation of the non-palpable sub-centimeter testicular lesion is depicted in Fig. 25.
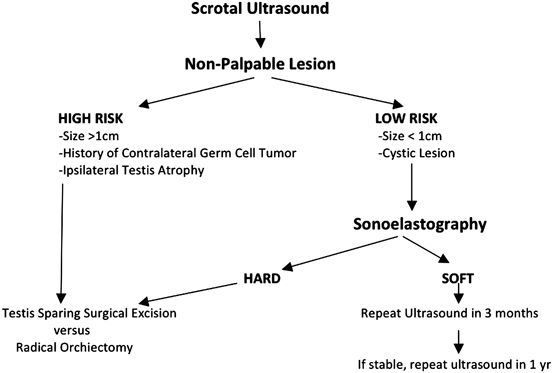
Fig. 25
Possible algorithm for management of a non-palpable testicular mass
Benign Abnormalities of the Testis
Torsion of the Spermatic Cord or Testicular Torsion
Ultrasound is often used to assess boys and adolescents with acute scrotal pain when the urologist is concerned for testicular torsion . Testicular torsion can be classified as extravaginal or intravaginal. The extravaginal form of torsion is found exclusively in newborn infants. Intravaginal torsion is more common and is due to a bell-and-clapper deformity in which the tunica vaginalis has an abnormally high insertion on the spermatic cord and completely encircles the testis, leaving the testis free to rotate within the tunica vaginalis. The deformity is bilateral in most cases. Intravaginal testicular torsion occurs most frequently in adolescent boys, with two thirds of cases occurring between 12 and 18 years of age. Intravaginal torsion may occur in testes that are retractile or are not fully descended. Blunt trauma , sudden forceful rotation of the body, or sudden exertion also predispose to testicular torsion .
Ultrasound is very effective in differentiating testicular torsion from other causes of acute scrotal pain. The severity of torsion of the testis can range from 180° to 720°, but complete occlusion of blood flow is thought to occur after 450° of torsion [11]. Transient or intermittent torsion with spontaneous resolution sometimes occurs. Venous congestion or occlusion progresses to arterial occlusion, testicular ischemia, and infarction. The collateral blood flow is typically not adequate to provide viability to the testicle if the testicular artery is occluded. There is a 90 % chance of salvaging the testicle when ischemia has been present for less than 6 h, which decreases to 50 % at 12 h and 10 % at 24 h [42]. While irreversible testicular damage is presumed after 4 h of torsion, only 50 % of men who were detorsed less than 4 h after their symptoms began were noted to have normal semen quality [43].
On gray scale ultrasound, the affected testis usually appears hypoechoic (Fig. 26a) and Doppler color flow study shows decreased or no flow in the affected testis (Fig. 26b). Testicular size can vary from increased to decreased when compared to its counterpart depends up on the duration of the torsion. The sonographer should always compare the affected testis with the contralateral side using longitudinal, transverse, and coronal views. When the sonographer attempts to align the transducer parallel to flow, apical views can be particularly informative. In patients with acute torsion, the epididymis may appear hypoechoic and enlarged, similar to epididymitis. With testicular torsion ultrasound may also demonstrate that the spermatic cord immediately cranial to the testis and epididymis is twisted, which gives it a characteristic “torsion knot” or “whirlpool appearance” (Fig. 27a, 27b).
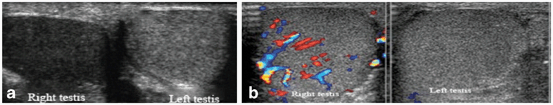
Fig. 26
a Right testicular torsion with normal left testis for comparison. The torsed testis has decreased echogenicity on gray scale ultrasonogram compared to the contralateral healthy testis. b Color Doppler ultrasound shows absent blood flow in the left testis with testicular torsion and normal flow in the healthy right testis
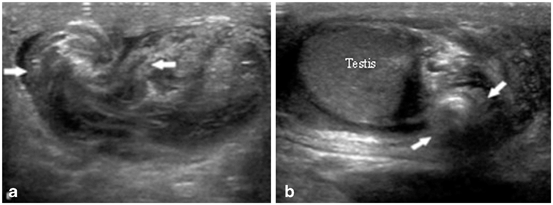
Fig. 27
a, b The spermatic cord immediately cranial to the testis and epididymis is twisted, which gives it a characteristic “torsion knot” or “whirlpool appearance” on gray scale ultrasound
Acute unilateral scrotal pain may be of a non-emergent etiology, due to epididymitis or torsion of a testicular or epididymal appendage. Waldert et al. retrospectively reviewed the charts of 298 boys who presented with an acute scrotum and underwent color Doppler ultrasonography followed by exploratory surgery, regardless of the sonographic findings. Twenty percent were diagnosed with testicular torsion, 56 % with torsion of an appendage, 8 % with epididymitis, and 11 % with no definite diagnosis. Color Doppler sonography sensitivity, specificity, positive predictive value and negative predictive value for testicular torsion were 96.8, 97.9, 92.1 and 99.1 % respectively. The two boys in this study misdiagnosed as epididymo-orchitis were both found to have 90° of torsion and no venous drainage but with residual arterial flow [44] .
Despite the findings that color Doppler sonography has a high sensitivity and specificity, it is our feeling that torsion remains a clinical diagnosis proven only at surgery. Ultrasound should only be used to document findings. Many conditions including torsion-detorsion, intermittent torsion, persistent capsular flow, and color flow artifacts can suggest apparent flow in cases where none exists. Therefore, ultrasound does not diagnose or “rule out” torsion, only surgical exploration is indicated when the diagnosis of testicular torsion is suspected .
Primary Orchitis and Testicular Abscess
The ultrasound findings of patients with orchitis are often an enlarged testis with homogenous appearance. Orchitis may be diffuse or focal, with focal orchitis appearing as multiple hypoechoic lesions with increased testicular blood flow (Fig. 28a, 28b). Additionally, the RI of the epididymal and testicular artery has been shown to be significantly lower in patients with epididymo-orchitis than in control subjects [45]. If inflammation progresses, the pressure of intratesticular edema may compromise blood flow leading to infarction; the ultrasound will demonstrate absence of blood flow and surrounding reactive hyperemia [46].
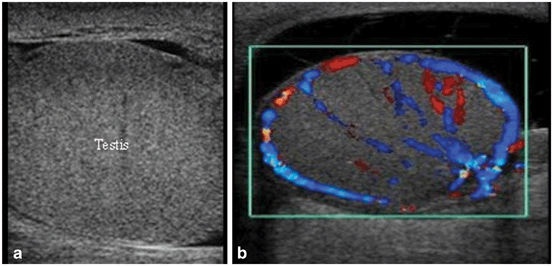
Fig. 28
a Gray scale ultrasound demonstrating orchitis with homogenous enlargement of the testis. b Color Doppler study shows increased blood flow to the testicle with prominent capsular vessels
A testicular abscess is seen in approximately 5 % of patients with orchitits and usually appears 1–7 weeks after orchitis, often as a result of ineffective treatment. The clinical hallmarks of a testicular abscess include persistent fever, scrotal pain and swelling. These findings may resemble a tumor, yet evidence of inflammation and absence of Doppler flow will often differentiate an abscess from a tumor [47] (Fig. 29).
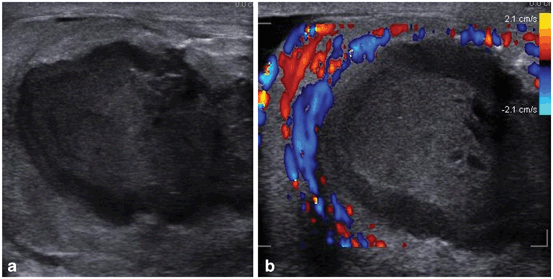
Fig. 29
Ultrasound findings shows heterogeneous complex septate fluid collection. Color Doppler ultrasound shows no blood flow in the abscess and increased blood flow in the surrounding testicular parenchyma
Nonpalpable Testis
When the testis is nonpalpable in the scrotum , a search is initiated to confirm its presence or absence . Ultrasound is often the initial diagnostic imaging modality because of its sensitivity in the inguinal canal where most undescended testes are found. If the absent testis is not identified within the inguinal canal, computerized tomography (CT) or magnetic resonance imaging (MRI) is can be used in an attempt to locate an intra-abdominal testis. Surgical exploration, however, remains the “gold standard” for identifying an intra-abdominal testis. A cryptorchid testis in the inguinal canal, identified by the presence of the mediastinum testis, is usually small in size (hypotrophic). It can be differentiated from an inguinal hernia by the absence of peristalsis and highly reflective omental fat .
Testicular Microcalcification
The etiology of testicular microcalcification (TM) is unknown. It has been suggested that the calcified concretions within the lumen of seminiferous tubules originates from sloughing of degenerated intratubular cells and failure of the Sertoli cells to phagocyte the debris [48, 49]. TM has been defined as five or more microcalcifications within the testicular parenchyma. TM appears on ultrasound as hyperechogenic lesions measuring between 1 and 3 mm sized multiple foci within the testicular parenchyma. The prevalence of TM varies from 1.5 to 5.6 % of asymptomatic healthy men, compared with 0.8–20 % in infertile men [50]. Acoustic shadowing on ultrasound is often absent, likely due to the small size of the calcifications [51, 52] (Figs. 30 and 31). They are usually bilateral, but occur unilaterally in 20 % of the cases. Goede et al. reported 2.4 % prevalence of TM in young asymptomatic boys [53]. TM is also described in association with various benign conditions including varicocele , cryptorchidism, male pseudo hermaphroditism, Klinefelter’s syndrome, neurofibromatosis, and Down syndrome [54].
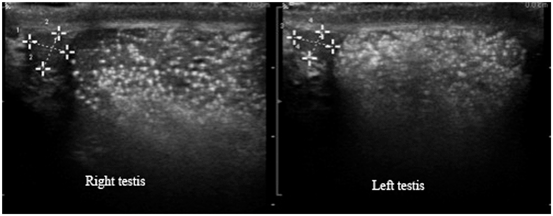
Fig. 30
Gray scale imaging demonstrating multiple bilateral microcalcifications of the testis. Note the lack of acoustic shadowing
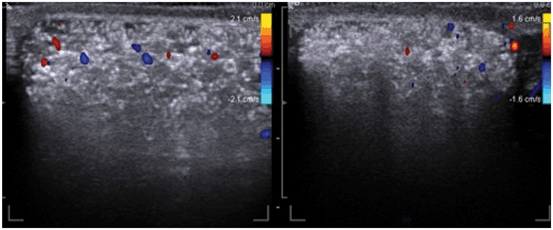
Fig. 31
Color Doppler study showing multiple microcalcifications
The risk of subsequent development of carcinoma in-situ (CIS) and testicular germ cell tumor in patients presenting with TM is less clear [52, 55]. Data from several investigators suggest that TM is a benign, nonprogressive condition, at least when followed for up to 45months [56, 57]. However, several recent case reports have documented the development of testicular tumors in patients with TM when follow up was extended for several years [57, 58]. The risk of CIS of testis in men with history of undescended testis is approximately 2–4 % [59]. Men with TM and associated risk factors should be considered for long-term follow up including testicular biopsy as indicated.
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree








