Sacral Nerve Stimulation
Klaus E. Matzel
Operative intervention for fecal incontinence should only be considered when conservative treatments have failed to result in adequate symptom relief.
The spectrum of indications for sacral nerve stimulation (SNS) is continually evolving. Since its first use for the treatment of fecal incontinence in 1994, its application has broadened. Initial indications were a very distinct population: patients presenting with fecal incontinence and residual function of a weak, but structurally intact, striated muscular anal sphincter and pelvic floor. However, the following findings widened the spectrum of indications:
The effect of SNS is not confined to the muscle relevant to continence.
Temporary test stimulation is low risk.
The result of a positive test stimulation is highly predictive of the clinical outcome of chronic therapeutic stimulation.
Today, test stimulation is liberally used, not only in established indications, but also to explore potential new indications, both for specific etiologies leading to incontinence and for other pathological conditions of the colorectum resulting in functional disorder. Permanent stimulation is directed by the clinical effectiveness of test stimulation.
Test stimulation is used on a pragmatic trial-and-error basis as it is clinically efficient and minimally invasive and because no other reliable clinical or physiologic predictor for a positive outcome of chronic SNS with a permanent neurostimulation device exists.
Patients are appropriate for test stimulation if they have existing, even if residual, voluntary anal sphincteric function or existing reflex sphincteric activity, indicating a nerve–muscle connection (confirmed by intact anocutaneous reflex activity, reflex contraction during sneezing or coughing, or a muscle response to pudendal stimulation with the St. Mark’s electrode).
Permanent stimulation with a fully implanted device is usually indicated if the trial stimulation results in >50% improvement symptom.
In addition to general contraindications (unfit for surgery or prone placement, bleeding diathesis), contraindications for test stimulation and implantation of the permanent device include the following:
Pathologic conditions of the sacrum preventing adequate electrode placement (such as congenital malformations)
Skin disease (especially septic) at the area of implantation
Micturition disorders that are considered contraindications for SNS
Pregnancy
Psychological instability, mental instability, or retardation that would impede understanding and handling the device programmer
The presence of devices incompatible with the implanted neurostimulator (cardiac pacemaker or implantable defibrillator)
The need for magnetic resonance imaging (MRI) in diagnosing or treating any other medical condition (the current generation of stimulation systems is not MRI-safe).
Preoperative planning is pragmatic and algorithmic. The decision making relies solely on documentation of the pretreatment bowel pattern and its change during temporary stimulation. It is helpful to know whether the patients retain voluntary sphincter/pelvic floor contractions or if reflex contraction can be provoked by a pin-prick test or coughing or sneezing. If both are missing, SNS is less promising.
Success of treatment (and of test stimulation) depends on appropriate electrode placement; preoperative sacral imaging in two planes will identify individual variances in bone anatomy and sacral foramina configuration. Preoperative bowel cleansing is not necessary.
For implantation of the permanent device, the position of the implantable pulse generator (INS) should be discussed with the patient and marked preoperatively. The patient must be able to reach it with the handheld programmer to activate and deactivate it or to change stimulation amplitude in a preset range. Interference with personal habits or clothing should be avoided.
Concept
Permanent SNS is indicated if trial stimulation results in symptom relief. Usually, a 50% reduction in the number of incontinent episodes or days with incontinence is considered adequate. The trial must be long enough to confirm these changes.
The SNS procedure consists of three steps:
In the first diagnostic stage, acute percutaneous nerve evaluation (PNE), the accessibility of the nerve/s through the sacral foramen and the feasibility of electrode placement are determined.
PNE assesses the relevance of each sacral spinal nerve to anal sphincteric contraction and anal canal closure/pelvic floor contraction. This information can help perform the following:
Distinguish between the true functional capability of the striated anal sphincteric muscles and the patient’s ability to make full voluntary use of them
Identify the individual pattern of peripheral innervation and demonstrate individual differences of the somatomotor/somatosensory innervation
Detect and determine the site of a possible lesion of the peripheral anal sphincteric nerve supply
Identify the optimal site for future stimulation
In the second diagnostic step, the therapeutic potential of stimulation is assessed by temporarily stimulating the sacral nerve identified during acute testing. As a therapeutic trial, it serves to select patients who may benefit from permanent neurostimulation.
In the third step, the aims is to improve symptoms permanently with continuous low-frequency stimulation.
PNE and permanent implantation can be performed under local or general anesthesia.
If general anesthesia is used, muscle relaxants must be avoided. They will suppress the motor reaction when the sacral nerves are stimulated and complicate identification of the optimal position for the electrode.
If local anesthesia is used, accidental blockade of the relevant sacral spinal nerves must be avoided, as the technique of electrode placement depends on a conducting nerve.
Anatomy
Technically, the most important part of the procedure at all stages—acute testing, subchronic test stimulation, and permanent implantation—is the appropriate placement of the electrode. It should be positioned close to the exit of the sacral spinal nerves through the ventral opening of the sacral foramen, at the site where the nerves enter the pelvic cavity and proximal to the formation of the sacral plexus.
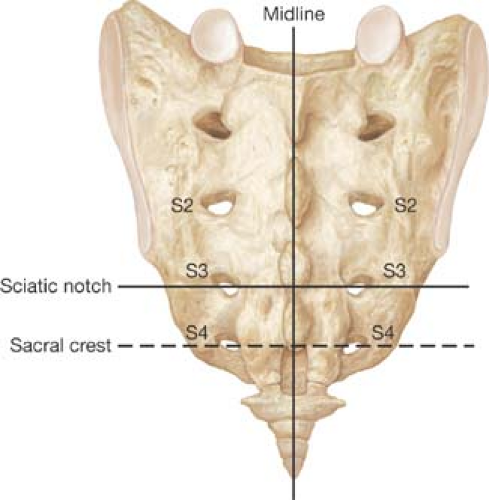
Distinct, palpable, bony anatomic landmarks help identify the sacral foramina. Most commonly, S3 is used for stimulation; during the procedure, it is also used as a reference site for orientation to place electrodes on S4 or S2.
The following landmarks help identify the foramina (Fig. 14.1):
The spinal processes mark the midline. Variations can occur, mostly distally.
The S3 foramen level is located medial to the upper edge of the greater sciatic notch.
The S3 foramen level (upper edge) corresponds to half the distance between the upper edge of the sacrum (lumbar–sacral junction) and the tip of the coccyx.
The S4 level corresponds with the sacral crest. Soft tissue coverage is least at S4 level.
The foramina are located 1–2 cm from the midline, which is marked by the palpable spinal processes. (The arrangement of the foramina relative to the midline may vary from parallel to a more V-shaped pattern.)
The distance between the levels of the sacral foramina is approximately 1.5 cm.
Neurostimulation Devices for SNS: Main Components
Test Stimulation Lead, Medtronic Model 3057: Unipolar lead designed to be implanted adjacent to the sacral nerve for temporary stimulation.
InterStim® Tined Lead, Medtronic Model 3889: A quadripolar in-line lead containing four cylindrical electrodes equal in length and spaced equidistantly. The lead has tines and marker bands. The tines anchor the lead, and the marker bands indicate lead depth and tine deployment during percutaneous implantation with a lead introducer.
InterStim® Tined Lead, Medtronic Model 3093: A quadripolar like Model 3889, but with a different electrode arrangement. The lead contains three cylindrical electrodes equal in length and spaced equidistantly and one extended (coiled) electrode approximately three times longer than the other three cylindrical electrodes.
InterStim® Implantable Neurostimulator, Medtronic Model 3023: A neurostimulator producing electrical pulses for stimulation with a variety of parameters, modes, and polarities. The implantable neurostimulator connects with an extension Model 3095, and the extension lead connects with a lead through which a stimulation program is delivered.
InterStim® II Implantable Neurostimulator, Medtronic Model 3058: Like Model 3023 above, a neurostimulator producing electrical pulses for stimulation with a variety of parameters, modes, and polarities. However, this is smaller, carries a smaller battery, and does not require a connecting cable to the electrode lead.
N’Vision Clinician Programmer, Medtronic Model 8840: Used by physicians with an 8870 Application Card (software) to program and communicate via telemetry with an InterStim or InterStim II neurostimulator.
iCon Patient Programmer, Medtronic Model 3037: To use with the InterStim (3023) or InterStim II (3058) neurostimulator. This handheld unit allows the patient to turn the neurostimulator on or off, change preset programs, adjust the amplitude within preset limits, and check the status of the neurostimulator and programmer batteries.
Patient Positioning
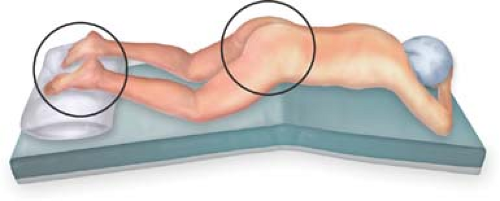
The patient is positioned prone (if fluoroscopy with lateral imaging of the sacrum is used on an X-ray-capable operating table).
The pelvis is elevated and supported. The legs and feet are fixed, but should be movable, as concomitant movements of the ipsilateral leg and foot during stimulation may aid electrode placement (Fig. 14.2).
The buttocks are taped to allow visual access to the anus and perineum. Taping should not be so tight as to counteract stimulation-induced contraction of the anus and the pelvic floor, which can be delicate.
The operative field (sacrum and buttock) is draped and sterile.
Visualization of a motor response of the anus and perianal area, as well as the feet, must be ensured.
Perioperative antibiotic prophylaxis is advised for implantation of permanent devices.
Acute Percutaneous Nerve Evaluation
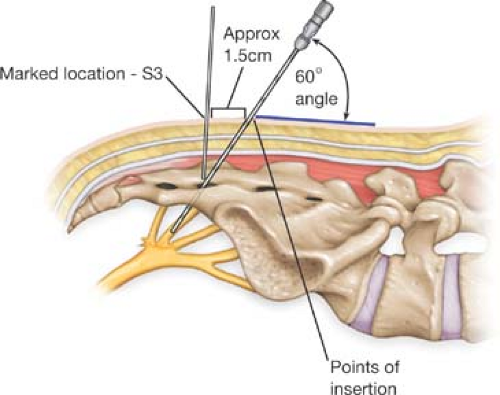
For acute PNE, needle electrodes (Medtronic Model 041828 or 041829 Foramen Needles), not isolated at the tip and top, are inserted into the dorsal sacral foramina of the
potentially relevant nerve—most commonly S3, but also S2 and S4. Placement is mainly guided by sacral bony landmarks and can be supported and confirmed by fluoroscopy.
potentially relevant nerve—most commonly S3, but also S2 and S4. Placement is mainly guided by sacral bony landmarks and can be supported and confirmed by fluoroscopy.
Identification of the sacral foramina: A distinct sensation of entering the dorsal opening of the foramen, perforating rigid ligamentous structures (as compared with hitting the periosteum of the sacrum), will be experienced.
Needle electrode positioning: The angle of insertion should be acute to minimize the risk of nerve or vascular damage, which is 60 degrees at the level of the skin (Fig. 14.3). As the needle has to cross soft tissue before entering the foramen, its entrance point should be cephalad to the position of the foramen.
Optimizing positioning: Once the foramen is entered, the needle electrode should be moved in a ventral direction (or back) with intermittent stimulation of graduated amplitude (beginning with low amplitudes). Gentle movements in millimeter steps with intermittent stimulation will help optimize positioning. Markers on the needle electrode indicate the depth of placement.
Response to stimulation: A motor response of the pelvic floor and the anus (if general anesthesia) or a sensory response (if local anesthesia) will optimize the positioning of the needle electrode.
Although the effect of stimulation on the pelvic floor and the lower extremity activity may vary among individuals, the following motor responses are generally typical:
S2 stimulation results in a clamp-like contraction of the perineal muscles and an outward rotation of the ipsilateral leg.
S3 stimulation leads to contraction of the levator ani and external anal sphincter, resulting in a bellows-like movement and circular contraction of the anus, along with plantar flexion of the first and second toes.
S4 stimulation produces a bellows-like contraction of the levator ani and circular contraction of the anus without movement of leg, foot, or toe.
Concomitant reactions of the leg/foot/toe can be observed, but are not essential. Their presence does not indicate a superior position of the electrode or a better clinical outcome.
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree