Fig. 10.1
Self anchoring tined lead
The system engages subcutaneous tissue, particularly muscle tissue, to decrease axial movement of the lead and consequent dislodgment of the stimulating electrodes.
The particularity of the tined lead is that the two-stage implant can be conducted in a fully percutaneous and simplified way.
Furthermore, this technique does not preclude other treatment options, and, in contrast to surgical interventions, it can be easily reversed. So it offers the advantage of a truly minimally invasive approach for SNM, performed under local anesthesia in an outpatient setting. As such, additional to fluoroscopy of radiopaque markers on the lead and motor responses (bellows contractions of the perineum, plantar flexion of the great toe), the patient’s conscious sensory responses (vaginal, perineal, or rectal) are accessible. These sensory responses are helpful to allow a more accurate placement of the permanent tined lead.
A positive outcome during screening with the tined lead was reported for 77–90 % of the tested patients [5, 6]. SNM with the tined lead resulted in permanent implant of the INS in significantly more urinary urge incontinent patients than with PNE (88 % vs. 46 %, p = 0.02).
The minimally invasive operative procedure to test and apply InterStim Therapy with the tined lead is performed with an insertion kit consisting of a foramen needle, a directional guide wire, a dilator with a concentric plastic sheath, and the tined lead [5]. The patient is placed in the prone position with a 45° flexion of the hips and knee joints. By using local anesthesia and intravenous conscious sedation, the foramen needle is inserted in the S3 foramina.
After ensuring correct sensory and motor responses, the inner stylet of the needle is removed and replaced with the directional guide. The foramen needle is then replaced by the dilator and introducer sheath, and thereafter the directional guide and dilator are removed, leaving the introducer sheath in position. Finally, the tined lead is inserted until the proximal electrode enters the foramen (Fig. 10.2).
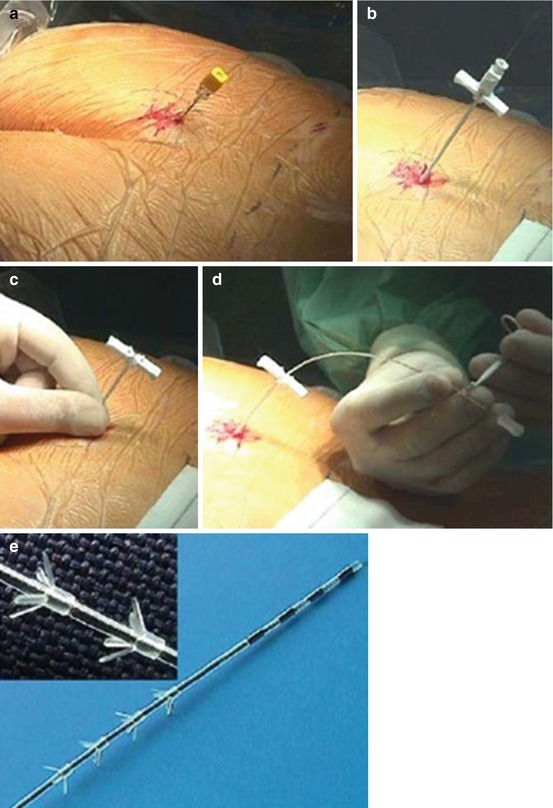

Fig. 10.2
Implant technique
To verify the lead’s position, an electrical signal is applied to evoke motor and sensory responses by the patient. While testing the electrodes, the position of the lead is also confirmed by fluoroscopy. With the lead held in place, the introducer sheath is retracted. The tined lead is tunnelled subcutaneously to the future implant pocket of the INS. Through a small incision at that ipsilateral place, an extension cable is introduced for connecting the tined lead subcutaneously to a pulse generator. The latter is situated at the contralateral side and is external during the first stage. This transposition with long tunnelling is chosen to prevent infection. In the second stage, the INS is implanted into the upper gluteal region in a subadipose pocket.
At the end of the each stage of the procedure and whenever there is a decrease in symptomatic response, it is recommended to perform sacral x-rays.
Buttock placement of the INS has become an alternative to subcutaneous implant in the lower part of the anterior abdominal wall because of the lower incidence of adverse events, shorter (approximately two times) operation time, and avoidance of patient repositioning during the operation.
In a prospective European multicenter study in 94 patients with different types of voiding dysfunction, screening with the tined lead was performed for on average 30 days [6]. This led to success in 72 patients (76.6 %) according to the physicians and at 6 weeks in 70 patients (74 %) when defined as ≥ 50 % improvement in symptoms compared to baseline; these 70 patients received the INS. After 6 months, follow-up data were available for 20 patients with UUI and 21 patients with UR. Patients with UUI had a significant reduction in the number of daily voids (p < 0.001), incontinent episodes (p < 0.005), and replaced pads (p = 0.0069). Patients with UR experienced improvements in the number of self-catheterizations (p < 0.001), voids per day (p < 0.001), and the catheterization volume (p < 0.001).
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree






