Reference
BRPC cases
Regiment
Number resected (%)
R0 resection (%)
OS, all pts (mo)
OS, resected pts (mo)
Turrini [85]
49
5-FU/Cis + XRT
9
b
b
b
Chun [24]
74
5-FU/Gem + XRT
74
44 (59 %)
b
23
Stokes [25]
40
Cape + XRT; Adj GEM
16 (46 %)
12 (75 %)
12
23
Barugola [86]
27
GEM +/− Cape/OX; then XRT + GEM +/− Cis/Cape
41
29 (71 %)
27.8
35
Katz [23]
129
GEM +/− Cis; GEM/5-FU + SBRT
85
81
22
32
Kang [87]
35
GEM +/− Cis + XRT
32
28
26.3
32.6
Chuong [26]
57
GTX; then SBRT
32 (56 %)
31 (97 %)
16.4
19.3
Kharofa [88]
39
Multiple
22 (56 %)
22
20.4
26.4
Paniccia [31]
20
FOLFIRINOX
17 (85 %)
17 (100 %)
b
b
Blazer [32]
18
mFOLFIRINOX
11
9
21.2
a
MDACC published one of the largest retrospective studies to date [23]. 129 patients were identified as borderline resectable by either MDACC or AHPBA/SSO/SSAT criteria; 70 met both sets of criteria. Patients were primarily treated with either sequential gemcitabine-based chemotherapy followed by chemoradiation or chemoradiation alone. A majority of patients underwent resection and nearly all achieved R0 resection. The average survival in the surgical group was 32 months while in the unresected population it was only 12 months. Benefit was seen even though preoperative therapy rarely resulted in clinically relevant downstaging of tumors. Importantly, this study shows benefit from chemoradiation independently or in sequence with chemotherapy.
Chun et al. [24] looked specifically at the impact of neoadjuvant chemoradiation on margin negative resection in borderline resectable cases involving the portal or superior mesenteric vein (PV/SMV). They compared 74 preoperatively treated patients to 35 that received upfront surgery. Of those treated, 78 % received gemcitabine-based chemoradiation while 22 % received 5-FU based chemoradiation. They found improved survival with chemoradiation in patients with unilateral involvement of the PV/SMV (Ishikawa type II and III); however, there was not a significant survival benefit with bilateral involvement (Ishikawa type IV and V). Overall, preoperative therapy and margin negative resection status both were associated with improved survival in these cases involving the PV/SMV.
Stokes and colleagues [25] evaluated patients with borderline resectable disease by the MDACC classification who were treated with preoperative capecitabine with radiation. Among the 40 BRPC patients, 85 % completed therapy and 16 underwent resection. R0 resection was achieved in 75 % of surgical cases. The authors conclude that capecitabine-based chemoradiation is well tolerated and effective in selecting patients most likely to benefit from surgery.
A single-institution analysis out of Moffitt [26] looked at sequential induction with 3 cycles of chemotherapy followed by SBRT in a cohort of BRPC patients. 66 % of patients received a combination of gemcitabine, docetaxel, and capecitabine (GTX) and the majority received gemcitabine-based therapy. Of those treated, 56 % went to surgery and 97 % of those achieved an R0 resection. Among these, three patients had a pathologic complete response (pCR) and one had a near pCR. These four patients were all treated with GTX and none had relapsed at time of publication. This result offers a suggestion that multi-agent chemotherapy regimens such as GTX may more effectively control micrometastatic disease than single-agent chemotherapy regimens.
An approach combining 5-FU, leucovorin, irinotecan, and oxaliplatin, collectively referred to as FOLFIRINOX, is an important treatment regimen that has become more common in the past several years. This regimen was developed for metastatic disease given from evidence that 5-FU plus oxaliplatin [27] and irinotecan [28] independently are effective in metastatic pancreatic adenocarcinoma. The regimen was reasonably tolerated with overall improvement in quality of life [29]. Subsequently, in a large phase III trial comparing FOLFIRINOX to gemcitabine, FOLFIRINOX was found to be superior in both progression-free and overall survival [30]. Of critical importance, FOLFIRINOX demonstrated a highly significant improvement in response rate, 31.6 % versus 9.4 % in the gemcitabine group. As a result, there is a strong desire to test the role of this regimen in the neoadjuvant setting.
Several small trials have reported positive outcomes with FOLFIRINOX in the preoperative setting. Paniccia [31] reported on a small retrospective cohort of patients who received FOLFIRINOX. Approximately half received only chemotherapy while the rest received chemotherapy followed by chemoradiation. In spite of expected toxicities, nearly 90 % of patients completed chemotherapy. 85 % underwent resection and all those patients achieved R0 resection. A separate study looking at a modified FOLFIRINOX regimen with reduced doses found similar rates of resection and high R0 resection rates with less toxicities [32].
While these retrospective studies yield valuable information, they have several limitations. Many early reviews have limit numbers of borderline cases or conversely do not explicitly define the criteria used to define patients as borderline resectable. Fortunately, more recent studies tend to have crisper definitions that make the resultant findings more application to this population. A major challenge is that most studies mix neoadjuvant treatment regimens. As such, it is difficult to determine what components of chemotherapy or chemoradiation are providing the most benefit. Fortunately, there are several small prospective trials to evaluate neoadjuvant regimens and several ongoing larger randomized controlled trials that are improving our preoperative chemotherapy regimens.
Prospective Studies with Preoperative Chemotherapy in BRPC, including Current Trials in Progress
Retrospective studies of borderline resectable disease provide a strong suggestion that patients benefit from presurgical chemotherapy. A variety of prospective trials with a borderline population have been conducted in the past decade to identify the most effective regimens and many trials are ongoing. First, we will highlight several prospective studies investigating the role of neoadjuvant chemotherapy as single-modality therapy in patients with BRPC.
Sahora et al. published the results of two separate phase II studies with neoadjuvant gemcitabine plus either oxaliplatin or docetaxel. In the gemcitabine and oxaliplatin (GemOx) study [33], patients received 6–9 weekly doses of GemOx with restaging and surgical exploration if evidence of response on imaging or clinically. Of the 15 patients who were classified as borderline resectable at enrollment, 47 % underwent surgical exploration. R0 resection rate was 69 %, and median survival was 22 months for resected versus 12 months for unresected patients. The gemcitabine and docetaxel (GemTax) trial [34] treated patients with 8 weeks (2 cycles) of GemTax prior to restaging. Patients with partial response or stable disease with improved clinical condition were taken for surgical exploration. Of the 12 patients with BRPC at study entry, 7 (58 %) underwent surgical exploration and ultimately 4 (33 %) were resected with curative intent. The overall R0 resection rate was 87 %. Median survival among resected versus unresected patients was 16.3 months versus 12.2 months, respectively.
A separate phase II study examined the role of neoadjuvant dose-dense gemcitabine and capecitabine (GX) in locally advanced pancreatic cancer [35]. Treatment typically consisted of 2 weeks of weekly gemcitabine and daily capecitabine on a 3-week cycle. Average number of treatment cycles was three. Per protocol, patients were classified as borderline resectable based on NCCN criteria and 18 BRPC patients were enrolled along with 23 unresectable patients. A total of 11 (61 %) underwent surgical resection and 9 of 11 (82 %) were R0 resections. Interestingly, the authors also analyzed patients based on Asian Pancreatobiliary Cancer Center (APBCC) criteria which results in 33 out of 43 patients being classified as borderline resectable. With broader inclusion criteria, a smaller proportion of patients (46 %) underwent resection, yet a greater number, 13 of 15 (87 %) were R0 resections. The median survival of resected patients was 23.1 months compared with 13.4 months in unresected patients. This trial also demonstrates the importance of standardization of BRPC criteria. The internal variability of results based on the borderline resectable classification system demonstrates the challenge of comparing results between trials.
Most centers use a combination of chemotherapy and chemoradiation therapy for neoadjuvant treatment of pancreatic adenocarcinoma, and many trials combine these modalities of treatment. Chemoradiation therapy can be delivered independently or sequentially with chemotherapy to treat BRPC. There is evidence supporting the use of 5-fluorouracil agents (infusional 5-FU or capecitabine) [36, 37] or gemcitabine [38, 39] as radiosensitizing agents in combination with radiotherapy. The NCCN notes that no standard chemoradiation regimen exists for the treatment of borderline resectable disease. Other chapters of this textbook will discuss the data supporting chemoradiation in greater detail.
Mehta and colleagues conducted the earliest prospective trials of preoperative chemoradiation in patients with borderline resectable characteristics. Specifically, they enrolled patients with pancreatic adenocarcinoma had greater than 1 cm of tumor abutment, but less than 180° involvement of the PV, SMV, or SMA [21]. Patients received protracted 5-FU infusion with concurrent radiation totaling between 50.4 and 56 Gy. Of those treated, 60 % underwent R0 resection and had a median survival of 30 months compared with 8 months for the remaining unresected patients.
A recent study by Takahashi et al. investigated a regimen of gemcitabine-based chemoradiation followed by gemcitabine in resectable and borderline resectable patients [40]. Of 80 BRPC patients, resection rate was 54 %, and among those resected 34 % were alive at 5 years. Notably, distant and peritoneal recurrence was significantly higher in the BRPC group than the baseline resectable cohort. In this study, neoadjuvant gemcitabine-based chemoradiation seems to be an effective therapy for resectable or BRPC. Given higher rates of recurrence, borderline resectable patients may benefit from higher intensity chemotherapy regimens in the neoadjuvant setting. Further trials investigating role of chemoradiation therapy are ongoing.
Subsequently, a large number of trials to evaluate the efficacy of neoadjuvant or preoperative chemotherapy in borderline or unresectable tumors have been conducted. A recent meta-analysis identified 57 studies that examined preoperative treatment with chemotherapy, radiation therapy, or chemoradiation in patients with unresectable tumors at diagnosis [41]. In their analysis—of patients with initially unresectable disease treated with neoadjuvant therapy—4.8 % achieved a complete response and 30.2 % achieved a partial response. These rates were higher with combination chemotherapy compared with monotherapy. Significantly, 33.2 % of patients underwent surgical exploration with resection; of these, 79.2 % of patients achieved an R0 resection. The profound implication of this meta-analysis is that preoperative therapy, with an emphasis on systemic therapy, offers the opportunity for a subset of patients previously considered incurable to be treated with curative intent. Importantly, the meta-analysis did not find a significant difference in the outcomes of patients initially presenting with resectable disease receiving neoadjuvant therapy compared with adjuvant. This highlights the importance of having criteria to identify which patients are unresectable and most likely to achieve R0 resection with neoadjuvant treatment.
There are multiple ongoing investigations into systemic neoadjuvant treatment options for BRPC. In addition to trying to find an optimal response rate, there is interest in achieving a balance of efficacy and toxicity. As many of these studies are in progress, it is premature to widely generalize results to broader patient populations; however, there are exciting signals for novel combinations and/or therapeutics.
Several early phase trials are investigating role of S-1, alone or in combination, for the treatment of BRPC. S-1 is an oral medication that consists of tegafur, a prodrug of 5-FU; gimeracil, a dihydropyrimidine dehydrogenase (DPD) inhibitor; and oteracil, an inhibitor of phosphorylation in the gastrointestinal tract. The prodrug is converted into active 5-FU via hepatic metabolism and degradation is blocked by inhibition of DPD. Two phase III studies conducted in Japan demonstrated non-inferiority to gemcitabine in the unresectable setting [42], and superiority alone or in combination with gemcitabine in the adjuvant setting [43, 44]. In one phase II trial, 35 BRPC patients were treated with combination gemcitabine and S-1. 27 had no evidence of distance metastatic disease at time of resection and had a median survival of 34.7 months compared with 10.0 months for those with unresectable or metastatic disease [45]. Another trial of chemoradiation therapy with S-1 enrolled 28 patients, 25 of whom completed treatment. 24 (85.7 %) underwent surgical resection and all achieved R0 resection. All resected pathology specimens had Evans’ grade IIa response, and 14 had grade IIb or greater reduction in tumor cells [46]. The large phase III trials of S-1 have taken place in Japan and there are concerns about how the toxicity profile, particularly in Western populations, may limit utilization of this drug [47]. These results are encouraging and trials of S-1 compared with the more aggressive and established neoadjuvant regimens are necessary.
Gemcitabine and nab-paclitaxel is an effective treatment for pancreatic adenocarcinoma in the metastatic setting. An early phase study of this combination demonstrated good tolerability and a promising response rate in the metastatic setting [48]. The IMPACT trial compared this regimen to gemcitabine alone and found a good improvement in response rate of 23 % versus 7 % in the experimental and control arms, respectively [49]. There are case reports describing the use of this regimen in the locally advanced setting with good response leading to resection [50]. Figure 6.1 demonstrates tumor and Ca 19-9 response in a patient treated with gemcitabine and nab-paclitaxel. Current ongoing trials are comparing these agents to FOLFIRINOX, in combination with radiation therapy, or even with novel targeted agents. Evidence-based data on the utility of this regimen in the borderline resectable population is under active investigation.
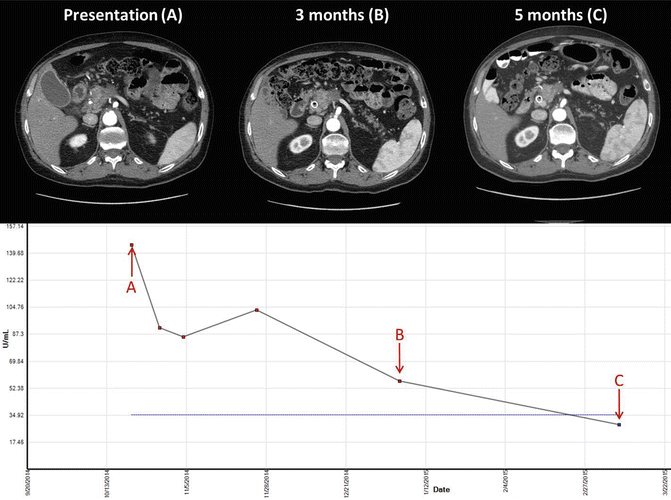

Fig. 6.1
Imaging and Ca 19-9 levels in a 67-year-old male patient with borderline resectable pancreatic adenocarcinoma treated with gemcitabine and nab-paclitaxel for 5 months followed by capecitabine-based chemoradiation therapy receiving a total of 50.4 Gy
FOLFIRINOX is being actively trialed in the BRPC population. Retrospective studies discussed earlier have shown much promise; however, validation from prospective trials is forthcoming. The Alliance A021101 intergroup trial for BRPC is a large trial to evaluate preoperative FOLFIRINOX followed by capecitabine-based chemoradiation and is currently ongoing. As an example, Fig. 6.2 demonstrates response to treatment without evidence of recurrence in a patient treated with FOLFIRINOX. Reduced intensity FOLFIRINOX is appealing as it seeks to combine the benefit of triple-agent therapy while minimizing dose-related toxicities. Retrospective studies have shown similar benefits to full dose therapy with fewer side effects [32]. However, prospective trials are needed. Several smaller prospective trials are investigating FOLFIRINOX in the resectable or locally advanced setting. A phase 2 trial at MDACC is investigating modified preoperative FOLFIRINOX and chemoradiation in high-risk resectable and borderline resectable pancreatic cancer patients. To date, using strict inclusion criteria for high-risk disease resection rates are approximately 50 %. While the data is still evolving, approximately 40 % of resected patients have demonstrated early recurrence at less than 1 year postoperatively. Importantly, it appears that aggressive therapy does not rescue aggressive biology in these patients. Therefore, better methods to identify the patients most likely to benefit from therapy are needed. NCT-01992705 is specifically investigating BRPC and combines this chemotherapy with SBRT; a similar trial, NCT-01897454, combines chemotherapy with gemcitabine-based radiation therapy. There is interest in combining immunotherapy modalities to target the immunosuppressive environment in pancreatic adenocarcinoma. NCT-01413022 is a phase Ib study combining FOLFIRINOX with a novel CCR2 inhibitor, an agent that has been shown to target infiltrating, inflammatory macrophages [51], demonstrated promising early results [52]. The results of multiple ongoing preoperative trials of FOLFIRINOX and other novel agents will provide a better understanding of the efficacy in achieving better surgical and long-term outcomes. Additionally, we need correlative blood based and tumor studies tied to these prospective trials that can serve as biomarkers and help separate responders and long-term survivors from patients who do poorly regardless of therapy plan.
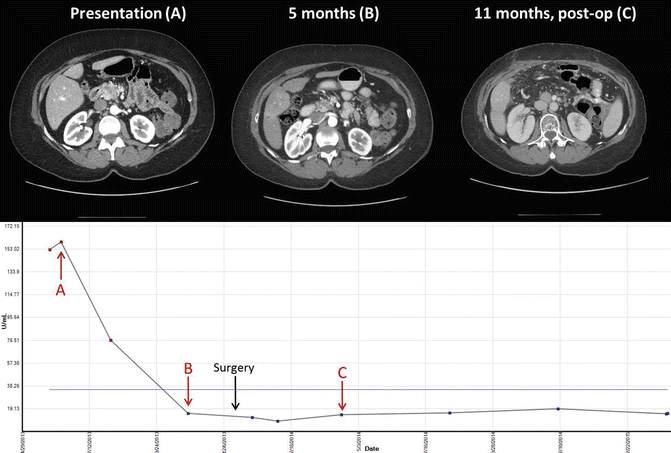

Fig. 6.2
Imaging and Ca 19-9 levels in a 51-year-old female with borderline resectable pancreatic adenocarcinoma treated with FOLFIRINOX for 2 months with dose reduction of oxaliplatin. This was followed by capecitabine-based chemoradiation with 50.4 Gy. CT images and Ca 19-9 levels include before and after surgical resection of primary tumor
There is not a well-established standard of treatment for BRPC. Therefore, a patient that is identified as having a primary cancer with questionable resectability or locally advanced disease that may meet criteria for BRPC should be considered for treatment on research protocol and/or referral to a high-volume center with experience in treating this subset of disease. If this is not possible, patient should receive some form of neoadjuvant treatment.
A recommended treatment algorithm is outlined below in Fig. 6.3. If on presentation, a patient presents with clearly resectable disease, they should proceed to surgery or receive preoperative treatment on protocol. If presenting with BRPC, they should receive some form of preoperative treatment based on their ability to handle and desire for aggressive treatment. Either FOLFIRINOX or gemcitabine-containing multi-agent regimens are reasonable treatment options.
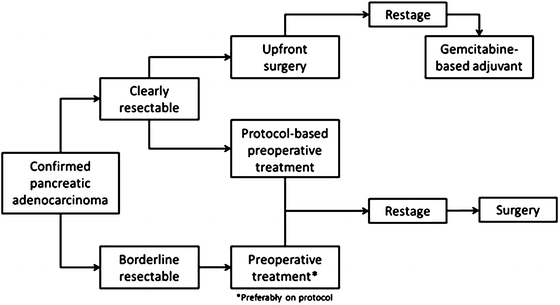

Fig. 6.3
Treatment algorithm
Rational Study Endpoints for Preoperative Trials
A challenge encountered in designing preoperative trials involves identifying the most relevant endpoints for a trial. Commonly cited outcome measures in studies include response rate, percentage of patients undergoing resection, and rate of margin negative resection. However, many of these features do not adequately predict relapse or overall survival. Typically, systemic therapy alone or in combination with radiation results in minimal reduction in the primary tumor volume. Therefore, criteria that qualify a response based on reduction in tumor volume are typically not a useful endpoint. Likewise, the percentage of patients who proceed to surgery after neoadjuvant treatment is heavily influenced by patient and tumor characteristics. Indeed, a major benefit of neoadjuvant treatment is to avoid early surgical intervention in patients with microscopically advanced disease that will declare itself as inoperable. At the present time, it is not to predict which BRPC patients have microscopic advanced disease at diagnosis. Therefore, it is possible that failure to undergo surgical resection may not represent failure of preoperative systemic therapy. Finally, margin negative resection seems to correspond well with survival in the majority of retrospective and prospective studies reported (Tables 6.1 and 6.2). Margin status most directly is representative of local disease control, but seems to act as an indirect surrogate for metastatic disease control.
Table 6.2
Prospective trials with neoadjuvant chemotherapy in BRPC
Reference | BRPC cases | Regiment | Number resected (%) | R0 resection (%) | OS, all patients (mo) | OS, resected pts (mo) |
|---|---|---|---|---|---|---|
Landry [89] | 21 | GEM + XRT versus GEM/Cis/5-FU then XRT | 5 | a | 16.4 | 26.3 |
Sahora [34] | 12 | GEM + Docetaxel | 8 (32 %) | 87 % | a | 16 |
Sahora [33] | 15 | GEM + OX | 13 (39 %) | 69 % | a | 22 |
Pipas [90] | 23 | Cetuximab + GEM + XRT | 25 (76 %) | 92 % | a | 24.3 |
Lee [35] | 18 | GEM + Cape | 11 (61 %) | 82 % | 16 | 23 |
Kim [91] | 39 | GEM + OX + XRT; Adj GEM + OX | 24 (62 %) | 84 % | 18 | 25 |
Motoi [92] | 16 | GEM + S1 (2c) | a | 87 % | 18 | a |
Takahashi [40] | 80 | GEM + XRT | 43 (54 %) | 54 % | 19 | 25 |
Rose [93]
Stay updated, free articles. Join our Telegram channel
Full access? Get Clinical Tree
 Get Clinical Tree app for offline access
Get Clinical Tree app for offline access

|




