AHPBA/SSAT/SSO
MD Anderson
NCCN 2012a
Intergroup trial
SMV-PV
Abutmentb, encasementc, or occlusion
Occlusion
Abutment with impingement or narrowing
Interface between tumor and vessel measuring 180° or greater of the circumference of the vessel wall, and/or reconstructabled occlusion
SMA
Abutment
Abutment
Abutment
Interface between tumor and vessel measuring less than 180° of the circumference of the vessel wall
CHA
Abutment or short-segment encasement
Abutment or short-segment encasement
Abutment or short-segment encasement
Reconstructabled, short-segment interface between tumor and vessel of any degree
Celiac trunk
No abutment or encasement
Abutment
No abutment or encasement
Interface between tumor and vessel measuring less than 180° of the circumference of the vessel wall
Prognostic Factors for Survival in Locally Advanced Pancreatic Cancer
Clinical parameters at time of diagnosis have long been demonstrated as prognostic factors in patients with locally advanced and metastatic pancreatic cancer. A representative analysis of 335 patients with histologically confirmed pancreatic cancer (36 % of whom had localized or locally advanced disease) showed poor performance status (ECOG PS 2–4) and weight loss >10 % were independently associated with shorter overall survival [4]. Similarly, a high Charlson age-comorbidity index >3 at presentation and weight loss >10 % during neoadjuvant chemoradiotherapy are independently associated with reduced survival after resection [5, 6].
More recently, perioperative serum carbohydrate antigen (CA) 19-9 has been demonstrated as a prognostic marker for outcome after definitive resection independent of adjuvant therapy [7–9]. CA 19-9 has also been shown to be a useful marker in the treatment of patients with metastatic pancreatic cancer [10, 11]. These developments have spurred interest in the incorporation of CA 19-9 monitoring in the neoadjuvant setting. In an analysis of 141 patients with borderline resectable pancreatic cancer treated with neoadjuvant therapy (NAT) at MD Anderson, 82 % experienced measurable decline in CA 19-9 over the course of NAT. Sixty percent of all patients underwent resection. The normalization of CA 19-9 after NAT was associated with longer median overall survival among both non-resected (15 vs. 11 months) and resected patients (38 vs. 26 months). In multivariate analysis, failure to normalize CA 19-9 was independently associated with reduced survival (hazard ratio 2.13). In a similar analysis reported by investigators at the University of Pittsburgh, following NAT, a CA 19-9 response of >50 % predicted for R0 resection (odds ratio 4.2) in a cohort consisting of 21 resectable, 40 borderline resectable, and 17 locally advanced presentations [12]. None of five borderline patients with an increase in CA 19-9 after NAT underwent R0 resection compared to 80 % of the remaining borderline resectable patients. Also, CA 19-9 response of >50 % independently predicted for improved survival (median overall survival 28 vs. 11.1 months).
Brief Review of Published Data on the Use of Radiotherapy in the Neoadjuvant Setting
Progress in the neoadjuvant management of borderline resectable pancreatic cancer has occurred in parallel with emerging data in the management of locally advanced and metastatic pancreatic cancer. In this section, relevant radiation therapy data are reviewed chronologically.
Early Investigational Approaches: External Beam Radiation as the Only Component of NAT (1970–1980s)
The rationale for neoadjuvant radiotherapy was first established in the 1970s after a seminal review of patterns of failure in patients treated with definitive surgery at Massachusetts General Hospital [13]. In 31 patients treated with radical surgery alone, 50 % had a local recurrence at time of death or last follow up. It was suggested from this analysis that radiotherapy after resection may improve cancer related survival and that preoperative radiotherapy could increase resectability. The feasibility of neoadjuvant radiotherapy was also established in the 1970s [14]. A representative early series of 17 patients reported successful radical operation in six patients after 40–50 Gy to the region of the pancreatic head. Analysis of resected specimens after preoperative radiotherapy showed severely degenerative cancer cells were more likely to be located at the advancing point of carcinoma providing a histopathologic basis for the theory of improving resectability [15]. The technique of preoperative radiotherapy gained institutional popularity in the 1980s [16]. One of the first published series reported on 54 consecutive patients deemed appropriate candidates for curative intent resection. Twenty-three patients were managed with neoadjuvant radiotherapy (50 Gy in 25 fractions with anterior-posterior parallel opposed portals to an average field size of 11 × 11 cm). These patients were compared to 31 patients who proceeded to immediate laparotomy. Although there was no difference in resectability between the two groups, 1 year survival was significantly improved and death due to regional recurrence within 1.5 postoperative years was less common (75 % vs. 43 %, p < 0.05). However, long-term survival was not affected [17]. In sum, these data suggest that after preoperative radiotherapy, a potential survival benefit from improved local regional control was overwhelmed by systemic failure.
Pre-gemcitabine-Based Chemoradiotherapy
Investigators at MD Anderson initiated a series of NAT protocols in the late 1980s. Technical feasibility and safety of this paradigm was established in 1992 with 28 patients with adenocarcinoma of the pancreatic head all of whom received preoperative radiotherapy (50.4 Gy) and concurrent daily fluorouracil [18]. All 28 patients completed the prescribed course of neoadjuvant therapy—five patients demonstrated metastatic disease at re-staging 4–5 weeks after completion of chemoradiotherapy and 23 underwent laparotomy. Six patients were not resected after laparotomy due to unsuspected metastatic disease found at laparotomy (three) or locally advanced unresectable disease (three). Seventeen patients successfully underwent pancreaticoduodenectomy with one perioperative death. The technical feasibility of electron beam intraoperative radiotherapy to the postoperative bed after pancreaticoduodenectomy was established soon thereafter [19]. The results of these two prospective studies laid the groundwork for a third prospective trial in which 39 patients received neoadjuvant chemoradiotherapy (30–50.4 Gy with daily 5-FU) and intraoperative radiotherapy (10 Gy with electron beam). Isolated local or peritoneal recurrences were documented in only 11 %, whereas 53 % developed liver metastases.
In parallel with the early prospective trials conducted at MD Anderson, investigators at Fox Chase performed a prospective feasibility study utilizing NAT prior to attempted resection of locally advanced pancreatic and periampullary carcinoma [20]. Thirty-four patients were treated with infusional 5-FU, bolus mitomycin-C, and radiotherapy (median 50.4 Gy). Twenty-five patients underwent exploration of whom 11 had liver or peritoneal metastases and 10 had potentially curative resections (R0 resections). Four of the 10 with potentially curative resections had previously unresectable disease based on laparotomy prior to neoadjuvant therapy. One patient died in the postoperative period. The promising results of this pilot study prompted an Eastern Cooperative Oncology Group phase II study of preoperative chemoradiotherapy for potentially resectable adenocarcinoma of the pancreas [21]. In this multi-institutional trial, 53 patients received NAT (50.4 Gy in 28 fractions with mitomycin 10 mg/m2 on day 2 and 5-FU continuous infusion days 2–5 and 29–32). Six patients developed distant metastases at re-staging after neoadjuvant therapy. Forty-one patients ultimately underwent surgery and six of these patients had local tumor not amenable for curative intent resection. Twenty-four patients underwent resection and the median survival for this group was 15.7 months.
After the feasibility and safety of 5-FU-based NAT was established for resectable patients, individual institutions began to systematically employ a neoadjuvant treatment paradigm for patients with questionably resectable disease. Many of the initial series investigating the role of NAT in the management of pancreatic cancer included 5-FU and mitomycin-C as radiosensitizers. A representative series from Stanford reported on 15 patients with “marginally” resectable adenocarcinoma of the pancreatic head (portal vein, superior mesenteric vein, or superior mesenteric artery involvement as identified by CT). Patients received external beam radiotherapy (50.4–56 Gy) with concurrent protracted venous infusion of 5-FU (250 mg/m2 per day). No patient experienced grade 3 toxicity during neoadjuvant therapy. Nine of the 15 patients underwent R0 pancreaticoduodenectomy [22]. The median survival for resected patients was 30 months. In 2001, investigators at Duke reviewed a series of 111 patients with localized pancreatic cancer treated with neoadjuvant 5-FU-based chemoradiotherapy (median 45 Gy) [23]. Tumors were defined as locally advanced with any arterial involvement or venous occlusion by computed tomography. The overall R0 resection rate was 72 and 19 % of patients with initially locally advanced carcinoma ultimately underwent resection.
Development of Gemcitabine-Based Chemoradiotherapy
In parallel with the development of neoadjuvant therapy protocols, gemcitabine gained popularity as an active agent in the management of advanced or metastatic pancreatic cancer and was shown in a randomized setting to prolong survival in comparison to 5-FU [24]. These data challenged the use of 5-FU-based chemotherapy in the management of locally advanced disease. In the same time frame, rapid technological advancements in the field of radiation oncology were occurring. Particularly, the advent of 3D conformal planning and intensity modulated radiotherapy (IMRT) allowed for the possibility of dose escalation while minimizing risk of acute and chronic gastrointestinal toxicity.
From preclinical trials, gemcitabine was known to be a potent radiosensitizer [25]. This prompted a series of phase I trials for locally advanced/unresectable pancreatic cancer which proved that moderate dose hypofractionated RT to conventional (historical) treatment volumes (regions of primary tumor and draining lymph nodes including para-aortic nodes) with concurrent full dose gemcitabine produced unacceptable rates of gastrointestinal toxicity [26–28]. Efforts at mitigating this toxicity took several approaches. One was to reduce both the volume of tissue irradiated and to reduce the number of fractions and dose per fraction of radiotherapy when using full dose gemcitabine. Using this approach, investigators at the University of Michigan recommended a dose of 36 Gy in fifteen 2.4 Gy fractions to gross tumor only. Subsequently these investigators extended this approach to 67 patients with locally advanced unresectable pancreatic cancer [29]. Of the 17 who ultimately underwent surgical exploration nine underwent resection (6 R0, N0).
This approach also provided the basis for a multi-institutional phase II trial in the early 2000s [30] in which 20 patients were treated with gemcitabine/RT with neoadjuvant intent (weekly gemcitabine 1000 mg/m2 on weeks 1 and 2, 36 Gy in 15 fractions (using 3D planning) with weekly gemcitabine 1000 mg/m2 on weeks 4–6, followed by weekly gemcitabine 1000 mg/m2 on weeks 8 and 9). Importantly, this is the first multi-institutional trial in which patients were prospectively evaluated for degree of resectability according to national or cooperative group guidelines. All 20 patients were deemed to have potentially resectable disease (six borderline resectable). Borderline cases were confirmed by endoscopic ultrasound or magnetic resonance imaging. 19 of 20 patients completed neoadjuvant therapy without interruption. Twenty underwent operative exploration of whom 17 were resected. The R0 resection rate was 94 % and at a median follow up of 18 months, 41 % remained alive and free of disease.
In contrast to early data with full dose gemcitabine, reduced dose gemcitabine was shown to be tolerable with more conventional radiotherapy treatment volumes and dose. In 2009, a large series from Germany reported on 120 patients with borderline or unresectable tumors [31], based on computed tomography by NCCN criteria. Patients received 55.8 Gy to the primary tumor and 50.4 Gy to regional nodes. A majority of patients received concurrent gemcitabine (300 mg/m2 on weeks 1, 2, 4, and 5) and cisplatin (30 mg/m2 on weeks 1, 2, 4, and 5). No subsequent chemotherapy was administered. 31.7 % underwent resection, among whom 95 % underwent R0 resection. Forty-seven percent had primary tumor downstaging (pathologic T stage in comparison to clinical T stage by computed tomography) and 24 % had upstaging. Median disease-specific survival for patients with R0 resection was 52 months in comparison to 11 months for patients with R1 resection.
The second multi-institutional prospective trial (E1200) of neoadjuvant therapy for prospectively defined borderline resectable pancreatic cancer was published in 2010 [32]. In this two-arm randomized phase II trial, patients with potentially resectable pancreatic cancer (defined as tumor abutting the portal vein or superior mesenteric vein, abutting the hepatic or superior mesenteric artery, extending to the origin of the gastroduodenal artery, or occluding the superior mesenteric vein <2 cm) were randomized to one of two NAT arms. In the first arm, patients received preoperative radiotherapy (50.4 Gy to gross tumor +2 cm margin with 3D planning) with concurrent gemcitabine (500 mg/m2 weekly). In the second arm, the same radiotherapy regimen was prescribed with concurrent gemcitabine, cisplatin, and 5-FU. In both arms, following surgery, maintenance therapy of gemcitabine 1000 mg/m2 for seven cycles was prescribed. Ten patients were enrolled in the first arm and 11 patients were enrolled in the second arm. Three patients in the first arm and two patients in the second arm underwent resection (total R0 resection rate of 60 %). Grade 4 toxicity was significant and more common in the first arm (36 % vs. 18 %). The median overall survival of resected patients was 26.3 months.
The largest single institution analysis of patients with systematically defined borderline resectable pancreatic cancer treated with neoadjuvant intent was performed by investigators at MD Anderson [33]. In this analysis, patients were separated into three types. Type A patients had borderline tumor anatomy with respect to regional vasculature based on CT (≤180° abutment of SMA or celiac axis, tumor abutment or encasement of a short segment of the hepatic artery, or short-segment occlusion of the SMV, PV, or SMV-PV confluence amenable to vascular resection and reconstruction). Type B patients had possible extrapancreatic metastatic disease (including those with biopsy proven N1 disease). Type C patients had marginal performance status or severe medical comorbidities. One hundred and sixty patients (7 %) of all patients diagnosed with pancreatic adenocarcinoma were classified as having borderline resectable disease based on these criteria. Eighty-four of these patients were type A and all received neoadjuvant chemotherapy with or without radiotherapy (50.4 Gy in 28 fractions or 30 Gy in 10 fractions using 3D planning with concurrent 5-FU, paclitaxel, gemcitabine, or capecitabine at radiosensitizing doses). Thirty-eight percent of these patients ultimately underwent resection with a 97 % R0 resection rate. Full dose concurrent gemcitabine was not used in this population. All resected patients had been treated with neoadjuvant chemoradiotherapy. Median survival for patients who underwent resection for Type A tumors was 40 vs. 15 months for those who did not undergo resection. Median overall survival of all 84 patients with Type A tumors was 21 months. Importantly, included in this population of patients was a cohort of borderline resectable patients treated with 4 field 3D conformal radiotherapy to 30 Gy in 10 fractions with large fields encompassing the regional nodes with concurrent reduced dose gemcitabine. Twenty-seven percent of these patients experienced severe toxicity (defined as prolonged hospitalization, GI bleed, more than 3 dose deletions of gemcitabine, discontinuation of 5-FU, or grade 5 toxicity). This cautionary experience highlights the importance of highly conformal radiotherapy (that is, reduced volumes of normal tissue irradiated, especially stomach and small bowel) even with reduced dose gemcitabine.
A recent prospective trial by Leone et al. in 2013 [34] demonstrated the feasibility of induction gemcitabine/oxaliplatin (GEMOX) prior to reduced dose gemcitabine and concurrent radiotherapy for patients with locally advanced and borderline resectable pancreatic cancer. Thirty-nine patients were enrolled in this study, of whom 15 had borderline resectable disease (by MD Anderson criteria). Patients received four cycles of GEMOX prior to re-staging in preparation for chemoradiotherapy. Twelve of 15 patients with borderline disease proceeded to chemoradiotherapy (50.4 Gy in 28 fractions using 3D planning). Nine of these 12 patients underwent resection and this group had a median overall survival of 31.5 months.
Advances in Radiation Delivery and the Integration of Full Dose Concurrent Gemcitabine-Based Chemotherapy
Given reports on the efficacy of full dose gemcitabine-based combination chemotherapy in the advanced setting, the importance of obtaining systemic control in the neoadjuvant setting was reinforced. A phase I study conducted at the University of Michigan showed the safety of the addition of oxaliplatin to full dose gemcitabine and radiotherapy for resectable and unresectable pancreatic cancer [35]. This prompted a multi-institutional phase II trial headed by Kim et al. [36] in which 68 patients with localized pancreatic adenocarcinoma were treated with gemcitabine/oxaliplatin and radiotherapy with neoadjuvant intent. Patients received two cycles of neoadjuvant gemcitabine (1000 mg/m2 on days 8, and 15) with oxaliplatin (85 mg/m2 on days 1 and 15) with 30 Gy in 15 fractions (to small fields encompassing a clinical target volume defined as gross disease +1 cm margin). Two cycles of adjuvant chemotherapy were given after resection. Resectability was defined radiographically according to NCCN criteria. Ninety percent completed the prescribed course of neoadjuvant therapy. At presentation, 23 patients had resectable disease, 39 had borderline resectable disease, and six had unresectable disease. Of the 39 patients with borderline disease, 30 underwent laparotomy and 24 underwent resection (62 %). Three of these 39 patients developed local progression on re-staging prior to surgery and one developed distant progression. Of note, of the 23 patients with NCCN resectable disease at presentation, two developed distant progression at re-staging prior to resection (8.7 %). The overall R0 resection rate was 84 % and 13 of 19 patients with SMA/celiac axis contact underwent R0 resection (68 %). Median survival for patients undergoing R0 resection was 34.6 months and median survival of 28 patients with borderline disease who underwent resection was 25.4 months. Of patients who underwent R0 or R1 resection, local recurrence as a component of first failure was 29 %. On multivariate analysis, incomplete resection (R1/2) was associated with decreased survival (HR 1.2).
In the early 2000s, IMRT became widely available. Advantages of IMRT over conventional 3D planning include highly conformal target coverage and sparing of adjacent organs allowing potential dose escalation and facilitating full dose, highly active systemic therapy with gemcitabine. Comparison of IMRT to 3D planning to illustrate these points is shown in Fig. 7.1.
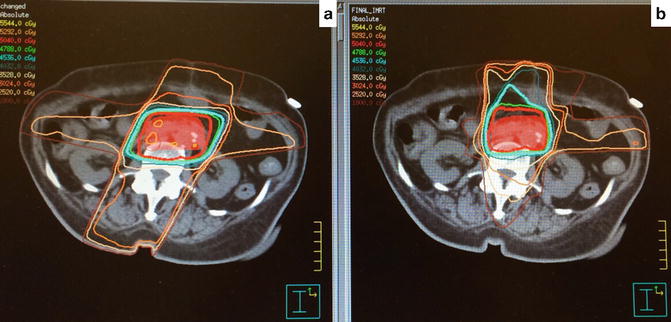

Fig. 7.1
3D conformal radiotherapy (a) vs. IMRT (b). Note increase in conformal target coverage and decrease in high dose to adjacent organs at risk
A phase I/II trial also conducted at the University of Michigan established the safety and efficacy of full dose gemcitabine and dose escalated IMRT in the locally advanced setting [37]. In this trial, a dose of 55 Gy in 25 fractions with concurrent full dose gemcitabine (1000 mg/m2 on days −22, −15, 1, 8, 22, and 29) was found to be the maximal tolerated radiotherapy dose with a 24 % probability of grade 3 or higher GI toxicity but also yielding an impressive 59 % 2-year local failure free survival.
Retrospective reports of the safety and efficacy of concurrent full dose gemcitabine-based chemoradiotherapy have also recently been published by others. Investigators at Osaka Medical center reported their retrospective results of NAT for borderline resectable pancreatic cancer in 2013 [38]. Of 268 patients treated with gemcitabine-based chemoradiotherapy, 80 had borderline resectable tumors (based on MD Anderson criteria). Patients received 50 Gy in 25 fractions to the primary tumor and regional lymph nodes using 3D conformal planning techniques and full dose gemcitabine (1000 mg/m2 weekly × three of every 4 week cycle for three cycles). 99.6 % completed the prescribed course of radiotherapy. For all patients, the most common toxicity was leukopenia (grade 3 47.4 %) and grade 3 GI toxicity was uncommon (3 %). Fifty-four percent of patients with borderline resectable disease underwent resection with 98 % R0 resection rate. Five-year survival for patients who underwent resection with resectable disease at presentation was 57 % vs. 34 % for patients with borderline disease. Although this represents a highly selected patient population, survival in this series compares favorably to patients treated with adjuvant chemotherapy alone in randomized settings. Nodal involvement and borderline resectability at presentation were significantly associated with poorer survival after resection on multivariate analysis. Importantly, adjuvant chemotherapy was not administered in this series.
Alternative concurrent chemotherapeutic agents: Although gemcitabine has gained considerable popularity as a concurrent agent during radiotherapy given its radiosensitization and highly active systemic properties, other radiosensitizers are being investigated. For example, Esnaola et al. reported on a phase II trial of induction gemcitabine, oxaliplatin, and cetuximab followed by capecitabine-based chemoradiotherapy for patients with locally advanced or borderline resectable pancreatic cancer [39]. Patients received six cycles of induction chemotherapy followed by chemoradiotherapy consisting of IMRT with a simultaneous integrated boost technique (45.9 Gy in 30 fractions to elective nodal regions and 54 Gy in 30 fractions to gross tumor). Daily concurrent capecitabine (800 mg/m2 BID) was prescribed on days of radiotherapy. Thirty-nine patients were enrolled and 69.2 % of all patients with borderline resectable disease (by NCCN criteria) achieved R0 resection.
Proton Therapy and SBRT in the Neoadjuvant Setting
An alternate approach to integrating radiotherapy and chemotherapy regimens not yet tested in the concurrent setting would be to use a very abbreviated course of radiotherapy sequentially with the intended systemic regimen. A five fraction regimen of accelerated hypofractionated radiotherapy has been shown to be effective in decreasing local recurrence in the neoadjuvant treatment of rectal cancer [40]. However, dose escalation and hypofractionation for pancreatic tumors are complicated by adjacent radiosensitive dose limiting structures such as the duodenum, stomach, small bowel, and kidneys. A dosimetric feasibility study at Massachusetts General Hospital showed that target coverage with proton beam radiotherapy was comparable to IMRT while mean dose (as a percentage of prescription dose) to kidney, liver, and small bowel were significantly improved [41]. This provided the impetus for a recently reported phase I/II trial investigating preoperative chemoradiotherapy for resectable pancreatic cancer with an accelerated hypofractionated course of proton beam radiotherapy [42]. The phase II dose was established at 5 daily doses of 5 Gy with concurrent capecitabine. Resected patients received adjuvant gemcitabine. There were two grade 3 toxicities and no grade 4 or 5 toxicities during chemoradiotherapy. Thirty-seven of 48 eligible patients underwent resection. Locoregional failure occurred in 16.2 % and distant recurrence occurred in 72.9 %. Proton beam radiotherapy as a component of neoadjuvant therapy for borderline resectable pancreatic cancer has not yet been reported.
Stereotactic body radiotherapy (SBRT) is a highly conformal treatment modality that requires precise diagnostic imaging capability, precise immobilization, and daily image guidance. Advantages include highly conformal treatment delivery with a sharp dose fall off in comparison to traditional 3D conformal or IMRT planning techniques (see Fig. 7.3). SBRT has been shown to be effective in obtaining local control in patients with unresectable locally advanced disease [43–45]. However, the use of SBRT in the neoadjuvant setting for borderline resectable pancreatic cancer remains investigational. Based on safety/tolerability and local control data for patients with unresectable disease, investigators have also examined the role of SBRT in the neoadjuvant setting. In 2013, Chuoung et al. reported on a series of 73 patients with unresectable or borderline resectable (by NCCN criteria) pancreatic cancer treated with induction chemotherapy followed by SBRT with neoadjuvant intent [46]. Median doses of 35 and 25 Gy were delivered to the region of vessel involvement and to the remainder of the tumor over five consecutive fractions. Thirty-two patients with borderline disease underwent surgery (56.1 %) and 31 achieved an R0 resection (96.9 %). These 31 patients had a median overall survival of 19.3 months. Late grade 3 toxicity was minimal (5.3 %). In 2015, Moningi et al. reported the Johns Hopkins experience on 88 patients with locally advanced and borderline resectable pancreatic cancer (defined according to SSO guidelines) treated with SBRT [47]. Seventy-four patients had locally advanced unresectable disease and 14 of these patients had borderline resectable disease. Patients received a total dose of 25–33 Gy in five fractions (PTV = GTV + 2–3 mm, gold fiducials placed in tumor). Institutional constraints for stomach and small bowel dose were employed. Most patients received pre-SBRT chemotherapy—19 patients ultimately underwent resection (79 % locally advanced at presentation) and 84 % had R0 resections. Resected patients had median survival of 20.2 months after SBRT. Late GI toxicity after SBRT was uncommon (5 % grade 3).
Molecular biology and selection of patients for neoadjuvant treatment with curative intent: Although R0 resection is universally accepted as a critical component of treatment with curative intent, the 5-year overall survival with completely resected pancreatic cancer remains poor (20–25 %). Resection after neoadjuvant therapy is associated with improved survival in two meta-analyses (see Tables 7.2 and 7.3). However, isolated locoregional recurrence is rarely a cause of cancer-specific mortality in this population. The tumor biology driving lymphovascular invasion and subsequent distant metastatic spread is believed to be distinct from the drivers for local growth. This concept is particularly relevant in the neoadjuvant treatment setting for borderline resectable pancreatic cancer and an understanding of tumor biology may, in the future, allow for the selection of appropriate patients a priori.
Table 7.2
Neoadjuvant chemoradiotherapy meta-analyses : resection is associated with improved survival
Morganti et al. [86] | Festa et al. [87] | |
|---|---|---|
Population | Unresectable at presentation | Borderline resectable at presentation |
Resection rate (%) | 27 | 55 |
Median survival of resected patients | 23.6 months | 22 months |
Long-term survival | 43 % at 3 years | 44 % at 2 years |
Median survival of unresected patients | 10 months | 10 months |
Table 7.3
Selected prospective neoadjuvant chemoradiotherapy trials for borderline resectable pancreatic cancer
References | Resectability criteria | Patients with BRPC | Pre-op regimen | Resection rate (%) | R0 resection rate (of patients resected) (%) | OS in patients with resected BRPC |
|---|---|---|---|---|---|---|
Kim et al. [36] | NCCN | 39 | Gem/Ox + RT (30 Gy) | 62 | 84 | 25 months |
Leone et al. [34] | MD Anderson | 15 | Gem/Ox → Gem + RT (50.4 Gy) | 60 | 82a
Stay updated, free articles. Join our Telegram channel
Full access? Get Clinical Tree
 Get Clinical Tree app for offline access
Get Clinical Tree app for offline access

|




