Fig. 14.1
Early postoperative hepatic artery thrombosis, which determined massive hepatic necrosis. The patient died awaiting re-OLT (red arrow: hepatic artery thrombosis)
On the other hand, late HAT, occurring more than 1 month after OLT, is a less common event and it may have a different evolution and outcome. This discrepancy in outcome between early and late HAT can be explained by the presence of arterial collaterals. After OLT, these collaterals (mainly derived from the phrenic arteries) are initially absent, but have been demonstrated angiographically as early as 2 weeks after OLT [40] (Fig. 14.2). Collaterals probably prevent biliary ischemic lesions in the case of late HAT [39, 40].
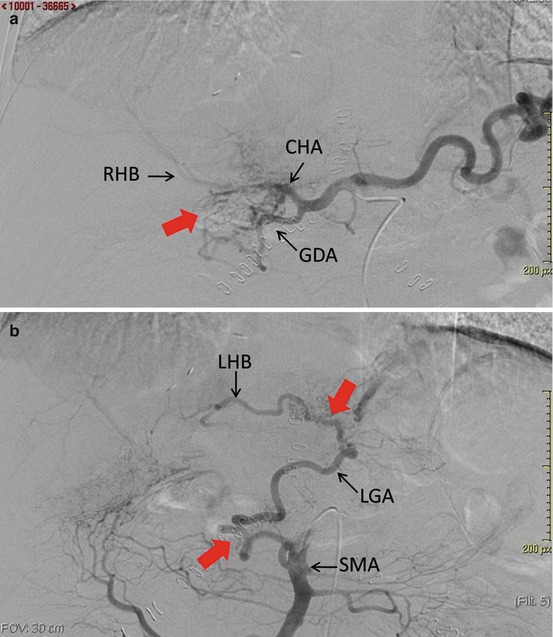

Fig. 14.2
Asymptomatic late hepatic artery thrombosis.(a) Selective angiography of the celiac trunk demonstrated common hepatic artery (CHA) thrombosis, with collateral arteries (red arrow) starting from the gastroduodenal artery (GDA), which revascularize the right hepatic branch (RHB). (b) Selective angiography of the superior mesenteric artery (SMA) demonstrated revascularization of the left gastric artery (LGA) and left hepatic branch (LHB) by collateral arteries (red arrows) starting from the SMA
Factors reported to reduce the incidence of eHAT include the use of microvascular surgical techniques, the use of Doppler ultrasound (DUS) immediately after surgery and daily scans for the first week, a low hematocrit, and the use of antiplatelet prophylaxis [31, 41]. The usual pattern of screening performed in our center is via routine DUS during the first postoperative week, while in cases of a suspicion of eHAT, a computed tomography angiography should be performed.
Various therapeutic options for managing HAT, including surgical revascularization [31, 32, 42, 43], portal vein arterialization [44], and endovascular treatments [45], are available, but re-OLT is still the treatment of choice for HAT [39]; in fact, alternative procedures (both radiological and surgical) to re-OLT can be complicated by the occurrence of biliary complications, among which biliary cast syndrome (BCS) is the most fearful. BCS, first described in 1975, is defined as the presence of a hardened, dark material within the biliary ductal system that takes the physical shape of the bile ducts [46] and is clinically characterized by fever, jaundice, and cholestatic liver enzyme elevation. Although endoscopic/interventional radiology techniques are successful and safe in the removal of biliary casts [47], BCS can ultimately cause substantial injury to the liver, with some transplant recipients requiring re-OLT (Fig. 14.3).
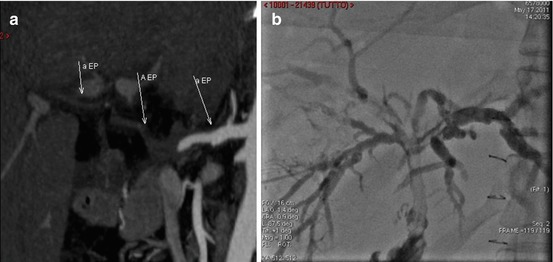

Fig. 14.3
(a) Early postoperative hepatic artery thrombosis, treated with surgical revascularization. (b) The patient developed biliary cast syndrome (note the multiple stenosis and filling defect of the biliary tree at cholangiography) that later required re-OLT (white arrows: hepatic artery course, prior to revascularization)
The 1-year patient and graft survival rates after re-OLT for eHAT are 20–60 % [38, 45, 48] and 50 %, respectively [49]. Asymptomatic eHAT detected by DUS and treated with early revascularization shows promising results [31, 32, 41–44]. It is, however, important to detect eHAT while the patient is still asymptomatic, because graft survival after revascularization is much better in this group compared to symptomatic patients (81.8 % versus 40 %, respectively) [50].
Portal vein thrombosis (PVT) is a rare but severe complication that typically occurs early after OLT and is often related to abnormal venous reconstruction during surgery in patients with preexisting PVT [51] or to the presence of spontaneous/surgical portosystemic shunts determining graft hypoperfusion [52]; its incidence ranges from 2.1 to 13 % [51–54]. When PVT occurs soon after OLT, urgent surgical management may be necessary for PV thrombectomy or the placement of interposition grafts because allograft survival and potentially patient survival may be negatively affected without restoration of PV flow. Other possible approaches to PVT are percutaneous thrombolysis, angioplasty, and stent placement. Re-OLT is rarely needed, especially in split-liver grafts/pediatric recipients experiencing early PVT, for the development of allograft failure [55].
Hepatic vein outflow obstruction after OLT is uncommon and occurs in less than 2 % of cases, but it is life-threatening if left untreated [56, 57]. The most common causes of hepatic vein thrombosis are anastomotic complications following OLT (30 %) [55]; although the majority of cases are managed conservatively with balloon angioplasty or transjugular intrahepatic portosystemic shunt placement, approximately 30 % of patients require re-OLT due to liver insufficiency [56].
14.3 Rejection
Nowadays, graft loss due to acute cellular rejection of the liver is an extremely rare condition, thanks to more effective immunosuppressive agents.
On the contrary, antibody-mediated rejection (AMR) has emerged as the cause of three types of rejection: (1) hyperacute rejection, (2) acute rejection, and (3) chronic rejection.
The hyperacute and acute forms of AMR are defined as acute rejection with graft dysfunction, histological evidence of acute tissue injury, and C4d deposition, in the presence of human leukocyte antigen donor-specific antibodies (DSA) [58]. The lack of interest in AMR in OLT is likely due to the fact that it is uncommon in liver recipients; however, in the last decade, a few cases of acute AMR after OLT have been reported [59]. O’Leary et al. have recently demonstrated that 5.8 % of previously unexplained early liver allograft loss was linked to AMR [60]; subsequently, the dosage of DSA is mandatory every time an unexplained graft dysfunction is present, in order to identify AMR early and promptly start the appropriate treatment, to avoid graft loss and the need for re-OLT [59–61].
Chronic rejection is the most common cause of late re-OLT [12, 19]. Thanks to advances in immunosuppressive therapy and to more sensitive means of detecting and diagnosing rejection, chronic rejection has considerably decreased in recent years as an indication for re-OLT, from 36 % at the end of the twentieth century to 9 % during the last few years [62].
14.4 Recurrence of Liver Disease
Hepatitis C virus recurrence accounts for 20–32 % of the cases of re-OLT, according to the most recent literature [7, 12–14, 62]. The indication for re-OLT for recurrent HCV-related allograft cirrhosis is still questionable, especially for the lower survival rate of these patients after re-OLT [7–9, 13].
However, the most recent literature agrees with the consideration that the timing of re-OLT performed for HCV recurrence is crucial to improve the outcome [13, 62, 63]. In particular, Marti et al. evaluated 108 patients who underwent nonurgent re-OLT adopting the Rosen score [19]. Only HCV-infected patients who developed cirrhosis at least 3 years after primary OLT underwent re-OLT. Applying these selection criteria, the authors did not find significant differences in survival after re-OLT at 1, 5, and 10 years between patients with hepatitis C recurrence (70 %, 57 %, and 57 %, respectively) and all other causes (72 %, 50 %, and 45 %, respectively) [62]. Moreover, McCashland et al., applying the Rosen score, reported 1- and 3- year survival rates after re-OLT of 69 and 49 % for HCV-infected patients and 73 and 55 % for non-HCV-infected individuals, respectively [63].
OLT is a well-accepted treatment modality for autoimmune liver disease. Recurrence of primary biliary cirrhosis (PBC), primary sclerosing cholangitis (PSC), and autoimmune hepatitis (AIH) after OLT has been widely described. While the impact of PBC recurrent disease on long-term survival after OLT is modest, with a re-OLT rate of 0.6–2 % [64, 65], PSC recurrence is very common, especially in the juvenile autoimmune form of sclerosing cholangitis (particularly if they have concurrent IBD), and it is associated with seriously compromised graft survival; in the case of PSC recurrence, a re-OLT rate of 5.4 % has been reported [66]. The AIH recurrence rate ranges from 12 to 46 % of cases [67]. Most patients with recurrent AIH respond to the reintroduction (or to an increase in dose) of corticosteroids and azathioprine, and re-OLT is a very rare event.
In conclusion, re-OLT is a high-risk procedure due to several early and late causes, weighted by an elevated rate of postoperative complications and a lower survival rate. In a context of organ shortage, it is mandatory to carefully select the patients who will benefit more from re-OLT, in order to avoid futile matches and graft loss.
References
1.
2.
3.
Busuttil RW, Farmer DG, Yersiz H, Hiatt JR, McDiarmid SV, Goldstein LI, Saab S, Han S, Durazo F, Weaver M, Cao C, Chen T, Lipshutz GS, Holt C, Gordon S, Gornbein J, Amersi F, Ghobrial RM. Analysis of long-term outcomes of 3200 liver transplantations over two decades: a single-center experience. Ann Surg. 2005;241:905–16.PubMedCentralCrossRefPubMed
4.
5.
Azoulay D, Linhares MM, Huguet E, Delvart V, Castaing D, Adam R, Ichai P, Saliba F, Lemoine A, Samuel D, Bismuth H. Decision for retransplantation of the liver: an experience- and cost-based analysis. Ann Surg. 2002;236:713–21.PubMedCentralCrossRefPubMed
6.
Doyle HR, Morelli F, McMichael J, Doria C, Aldrighetti L, Starzl TE, Marino IR. Hepatic retransplantation – an analysis of risk factors associated with outcome. Transplantation. 1996;61:1499–505.PubMedCentralCrossRefPubMed
7.
8.
9.
10.
Rosen HR, Madden JP, Martin P. A model to predict survival following liver retransplantation. Hepatology. 1999;29:365–70.CrossRefPubMed
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree






