Fig. 13.1
(a) Cholangiogram shows a fistula at the site of the biliodigestive anastomosis, with an associated collection (biloma) as demonstrated on CT scan (b). The cholangiographic follow-up, performed 20 days after biliary drainage insertion and external drainage positioned under CT guidance into the biloma, shows reduction of the leakage (c). The cholangiographic follow-up at 40 days, after external drainage removal, shows resolution of the fistula, and therefore the biliary drainage was removed (d)
In biliary steno-obstructions, after having crossed with a guidewire the stenotic bile duct, high-pressure angioplasty-type balloon catheter (bilioplasty) is inserted, chosen on the basis of the location of the stricture and the diameter of the normal bile duct (range from 6 to 12 mm). Usually, the balloon is inflated two or three consecutive times at high pressure, for 1–3 min during the same session, and the success of dilation is defined as the disappearance of a balloon waist during inflation. After dilation, a transhepatic biliary drainage of adequate caliber is left in place across the stenosis, as a protection from restenosis during the healing process. The patient is then discharged and returns as an outpatient for follow-up cholangiography, repeated dilation, and to replace the biliary catheter at 2- to 3-week intervals. In most cases, more subsequent sessions of balloon dilations are required (mainly in anastomotic strictures) upsizing the balloon catheters, before the morphological and functional results become stabilized, while the biliary catheter has to remain in place for several weeks or months (Figs. 13.2 and 13.3). In the presence of complex strictures, multiple accesses may be required to place two or more catheters. The presence of sludge, stones, or bile casts upstream of the stenosis requires their removal through the use of occlusion catheters [8] or Dormia baskets, with immediate success in 60–90 % of cases.
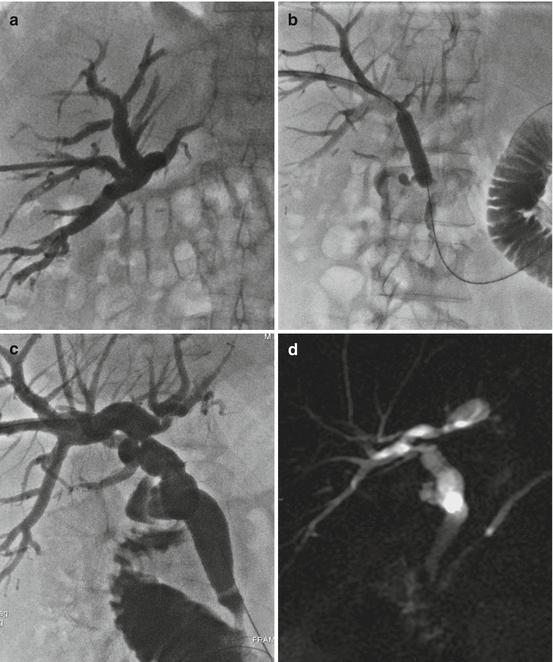
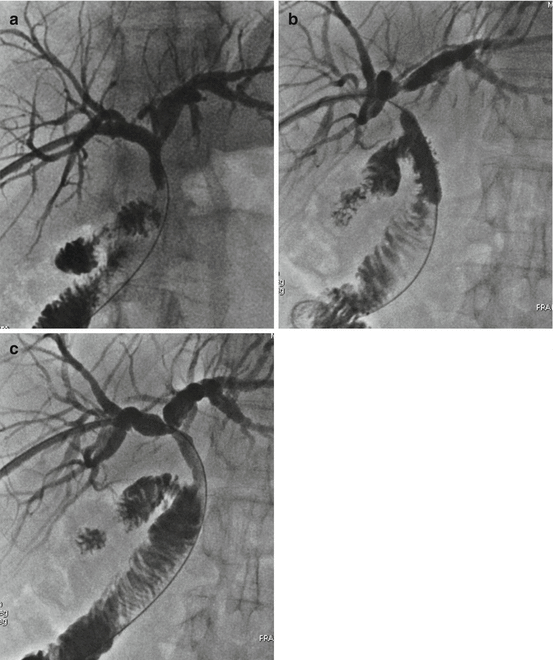

Fig. 13.2
(a) Percutaneous transhepatic cholangiography shows biliary stenosis at the site of the biliodigestive anastomosis. (b) The stricture was treated with balloon dilation. (c) Cholangiography performed after three bilioplasty sessions show complete resolution of the anastomotic stenosis. (d) Magnetic resonance cholangiogram 3 months after the completion of treatment confirms the stability of results

Fig. 13.3
(a) Percutaneous transhepatic cholangiography shows an almost complete obstruction at the site of the choledochocholedochal anastomosis associated with intrahepatic biliary duct dilatation. The patient was treated with repeated balloon dilation of the anastomotic stricture (b). At the end of the treatment, cholangiography shows restored patency of choledochocholedochal anastomosis (c)
Among different variables in the percutaneous transhepatic balloon dilation protocols, none have proven to improve long-term patency. Success rate varies between 70 and 90 % at 3–6 years, with restenosis at 1 year in 20 % of cases [8, 13–15] depending on the technique. Nonischemic stenoses provide better results in less repeated sessions, whereas ischemic stenoses require a closer follow-up and multiple repeated bilioplasty sessions, but they obtain better secondary patency at 3 years [12].
In refractory biliary strictures with recurrence after balloon dilatation or surgical repair, it is possible to place self-expanding stainless steel stents, which exert a continuous outward radial pressure on the bile duct and prevent the elastic recoil of the wall. Stent positioning has a reported technical success rate approaching 100 % and 3-year patency rate of more than 90 % [16]. Placement of the recently introduced covered removable stent across stenosis is an alternative option in cases of recurrence after repeated bilioplasty. The optimal removal period is about 6 months; after 9 months, removal becomes more difficult as it increases the risk of complications. Another possible complication is their migration due to lack of stability.
If the dilation causes hemobilia, a biliary drain should be left in place for a few days to prevent blood clots from creating a possible occlusion of the lumen.
Post-LT percutaneous procedures may incur in a series of complications (8–30 %) such as hemobilia, bleeding with subcapsular hematoma formation, cholangitis, pancreatitis, and fistulas due to biliary or duodenum perforation, usually spontaneously resolving [12, 16–18].
Sphincter of Oddi dysfunction, also termed ampullary dysfunction, occurs in 3–5 % of LT recipients and presents with cholestasis, dilatation of the distal bile duct, and cholangiography failing to detect any anatomic cause for biliary obstruction. It may be caused by operative denervation of the sphincter of Oddi during recipient hepatectomy, leading to subsequent impairment of ampullary relaxation and increased intraductal biliary pressure. The diagnosis may be confirmed clinically by decreasing cholestasis with T-tube unclamping, delayed drainage of contrast medium after cholangiography, and manometry [1]. Endoscopic sphincterotomy and biliary stenting are usually successful treatments, but conversion to a hepaticojejunostomy may occasionally be required.
13.2 Vascular Complications
Vascular complications after LT have a mean prevalence of 9 % and a wide variability among series (2–25 %), but are the most frequent cause of graft failure. They can be classified as early (within 3 months after LT, mostly including hemorrhages, thrombosis, or stenosis) or late (beyond 3 months, mainly comprising stenosis, thrombosis, and pseudoaneurysms) [19, 20]. The most frequent and critical vascular complications involve the hepatic artery and consist of hepatic artery stenosis and thrombosis, whereas portal and hepatic venous complications are less common and include stenosis and occlusion of the portal vein, hepatic veins, and inferior vena cava (IVC) [21–24]. In a series of 429 patients [21], arterial complications accounted for 6 % – including arterial thrombosis (58 %), stenosis (31 %), kinking (6 %), and pseudoaneurysms (5 %) – portal vein complications accounted for 1 %, and IVC and hepatic veins abnormalities for 2.5 %. In all cases, two therapeutic options, surgical or percutaneous, can be considered.
13.2.1 Arterial Complications
Hepatic artery thrombosis (HAT) is the most feared vascular complication, with incidence of 4–15 % in LT; its usual site is at the anastomotic level, with onset variable from weeks to months following LT, being more frequent during the early post-LT period [20]. Re-transplantation is required whenever thrombolysis and surgical thrombectomy do not allow salvage of the graft. Early HAT, appearing within 1 month after LT, more frequently needs a surgical approach of revascularization – if the graft function is still maintained – as an alternative of re-transplantation. Late HAT (after 1 month) can be treated more conservatively, by managing secondary ischemic complications such as biliary necrosis (treated with percutaneous biliary drainage) or parenchymal breakdown and abscess formation (treated with percutaneous abscess drainage). The significance of HAT stems from the relationship of the hepatic artery with the biliary epithelium; since the hepatic artery is the sole blood supply to bile ducts, its compromise can quickly lead to biliary ischemia, necrosis, bilomas, and biliary stricture onset.
The endovascular treatment of HAT should include intra-arterial thrombolysis (safely performed since 1–3 weeks after LT), currently achieved by combining mechanical thrombolysis (thrombus maceration) of the intra-arterial thrombus and pharmaceutical thrombolysis (infusion within the thrombus through a multi-perforated catheter) by using urokinase or recombinant tissue plasminogen activator (r-tPA, Alteplase). In conjunction with intra-arterial thrombolysis perfusion, peripheral intravenous heparin is infused to prevent pericatheter thrombosis [25, 26]. Interval angiography after 12–24 h of thrombolysis is performed to assess the progress of the thrombolysis process and, if flow has been reestablished, assess for underlying anatomical defects such as arterial stenosis or kinking, to be treated consequently. Definitive success is defined as resolution of the thrombus without arterial anatomical defects reducing the arterial diameter lumen more than 50 % after 36–48 h of thrombolysis (Fig. 13.4). This treatment is burdened with bleeding complications, especially when performed in the early stages post-LT [27].
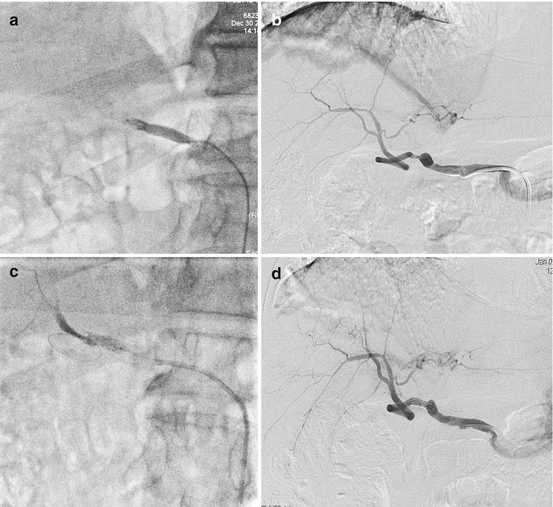

Fig. 13.4
(a) Selective hepatic arteriography shows complete thrombotic obstruction of the common hepatic artery at the surgical anastomosis with onset 3 months following LT; (b) the angiographic follow-up after 48 h from administration of urokinase showed resolution of the thrombus with residual stenosis, treated with deployment of a stent at the anastomotic level (c); final angiographic study demonstrates regular stent patency with preserved downstream arterial flow (d)
Hepatic artery stenosis (HAS) affects up to 11 % of transplant recipients and usually occurs at the anastomosis. The average onset of clinically significant stenosis is approximately 3 months and is more common in patients with a history of surgical clamp injury – responsible for proximal stenosis at the anastomotic site – and rejection, which commonly appears with multiple intrahepatic artery involvement [26]. Biliary sequelae can be observed in over 60 % of cases, with diffuse intrahepatic ducts involvement in up to 42 % [28]. Percutaneous endoluminal procedures are the first choice for HAS treatment, having less morbidity than surgery. HAS can be treated by percutaneous transluminal angioplasty (PTA), which must be performed at least 35 days after transplantation to prevent anastomotic lesions and bleeding [29]. This procedure, after intravenous administration of heparin, encompasses the selective catheterization of the involved artery, followed by the crossing of the stenotic segment with a guidewire on which an angioplasty double-lumen balloon catheter is advanced, placed over the stenosis, and inflated. Balloon size is determined by direct measurement of the patent portion of the hepatic artery at imaging (usually angio-CT). Possible complications of PTA (7–10 %) include hepatic artery rupture/perforation, thrombosis, dissection, and spasm [26, 30, 31]. Failure of the procedure is defined by relapse of stenosis, reported in 32–40 % of cases [26]. In case of recurrence, mainly due to longer segment stenosis (>3 cm) or after occurrence of parietal tears, a trans-stenotic metallic stent positioning is recommended [29] (Fig. 13.4).
Pseudoaneurysm of the hepatic artery is a rare, but potentially catastrophic, complication of LT requiring prompt treatment. While most pseudoaneurysms are asymptomatic and discovered incidentally during surveillance imaging, rupture of the pseudoaneurysm may present with peritoneal signs, gastrointestinal hemorrhage, hemobilia, hypotension, or death. Extrahepatic pseudoaneurysms most commonly arise at the anastomosis and can be a sequela of PTA, while intrahepatic pseudoaneurysms may be secondary to infection, biopsy, or biliary interventions. Technological improvements of angiographic materials have led to fast, safe, and effective embolization procedures, which have replaced emergency surgery as the first treatment choice. Permanent materials including, plugs, metallic coils, or liquid materials (e.g., isobutyl cyanoacrylate [glue], polyvinyl alcohol [PVA]) serve to occlude proximal or distal vessels, and covered stent grafts are usually used for proximal large artery rupture and pseudoaneurysms. In pseudoaneurysms of the hepatic artery, elective percutaneous treatment differs depending on its proximal or distal location: if the pseudoaneurysm occurs in the intrahepatic tract, endovascular embolization of the afferent branch of the aneurysm sac is performed by using micro-coils or permanent liquid embolic material. If the pseudoaneurysm is located in the extrahepatic tract, placement of a metallic covered stent graft is generally preferred to exclude the aneurysm sac from the main bloodstream.
Arterioportal fistula is a common transient phenomenon that can be detected in up to half of patients undergoing percutaneous biopsy. Most of these fistulas resolve spontaneously, with only approximately 10 % remaining past the first week after biopsy. A superselective embolization is the elective treatment by using appropriate angiographic material according to the fistula location [29, 31, 32].
Hepatic arterial kinking occurs in about 0.4 % of LT, and 7 % of HAS are associated to arterial kinking. The causes are due to donor or graft arterial redundancy or, more rarely, to external compression from surgical drainages. Surgical repair is the first choice, and, whenever not feasible, endoluminal correction can be attempted by stent positioning in order to straighten the vessel segment.
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree






