Reporting
Judy Yee
Effective communication of the results of imaging studies is an important part of radiology. Results of imaging studies should be communicated in a timely manner so that appropriate modifications in patient management may be made when necessary. The American College of Radiology (ACR) has published practice guidelines for the communication of diagnostic imaging findings.1 Suggested components of the report include demographic information such as location of where the study was performed, patient identification, referring physician versus self-referred, and name and date of the study. Relevant clinical information should be included as available. The body of the report should include a description of the procedure and materials, complications encountered, and findings of the study. Potential limitations and clinical issues should be addressed in the report. Comparison with prior relevant studies should be made and also reported.
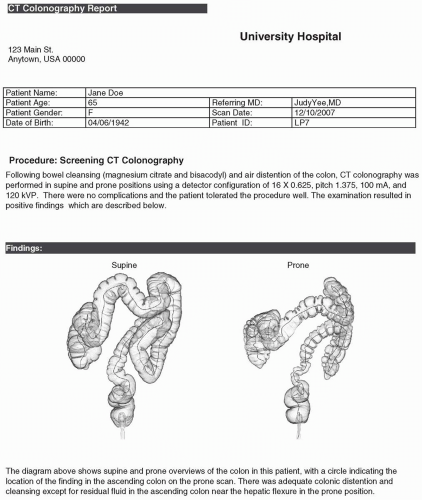
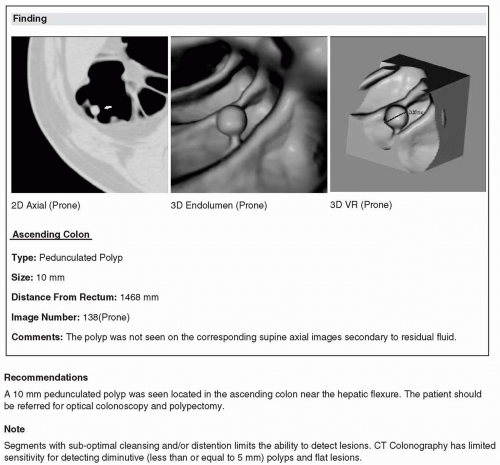
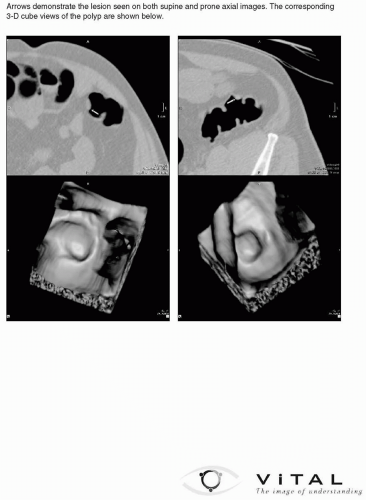
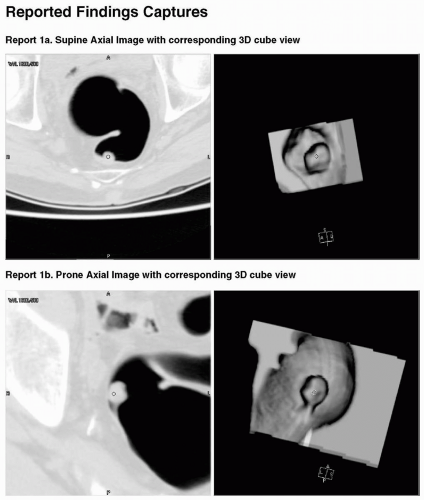
The documentation of the findings made on computed tomographic colonography (CTC) in a report must be accurate and complete. CTC reports share an overall similar format to other radiology reports. The ACR Practice Guideline for the performance of CTC in adults states that reporting should be in accordance with the ACR Practice Guideline for Communication of Diagnostic Imaging Findings.1,2 There are also certain elements of the report specific to CTC that are discussed in the subsequent text (see Figures 9.1, 9.2, 9.3, 9.4, 9.5 and 9.6). Template reports that contain the needed elements of CTC documentation are useful and an efficient method of reporting findings.
IDENTIFIERS
Basic demographic information included in the radiology report is patient name and another identifier. Patient gender, birth date, or age may be stated. The name and address of the site where the CTC is performed are included. The name and date of the examination are stated. The ACR Practice Guideline for Communication of Diagnostic Imaging Findings also encourages inclusion of the date of dictation and the date and time of transcription of the report.1
METHOD OF REFERRAL
The name of the physician or health care provider who refers the patient for CTC is recorded. The inclusion of contact information such as the phone number of the physician or health care provider makes it easier and more efficient for the communication of significant findings. If the patient is self-referred then that should be stated. Some states do not allow patient self-referral for CTC. The ACR Practice Guideline for Communication of Diagnostic Imaging Findings states that diagnostic imagers should recognize that performing imaging studies on self-referred patients establishes a doctor-patient relation that includes responsibility for communicating the study results directly to the patient and arranging for appropriate follow-up.1
PATIENT HISTORY
Clinicians should be encouraged to provide relevant clinical information. If the patient is symptomatic then the specific symptoms for which the CTC is being obtained should be included such as abdominal pain, diarrhea, constipation, weight loss, possible bowel obstruction, hematochezia, bright red blood per rectum, positive fecal occult blood test, and/or iron deficiency anemia. Pertinent patient history such as personal or family history of prior polyps or colorectal carcinoma should be included. Patients with one first-degree relative (mother, father, brother, sister, or child) with colorectal neoplasia before the age of 60 or who have multiple relatives who develop colorectal neoplasia at any age are considered to be at increased risk.2 Individuals who are asymptomatic but with a history of prior benign or malignant colorectal neoplasm undergo CTC as a surveillance examination and this should be noted. If the patient undergoing CTC for screening is asymptomatic and at average risk for colorectal carcinoma, then this is stated.
Patients undergoing CTC because of a failed or incomplete colonoscopy should have this documented with the portion of the colon successfully evaluated by
colonoscopy recorded if known. If the reason for the failed colonoscopy is identified before the CTC then this should be noted.
colonoscopy recorded if known. If the reason for the failed colonoscopy is identified before the CTC then this should be noted.
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree