Fig. 22.1
CT scan: lymphocele near the right renal graft in a double kidney transplant recipient
The treatment consists of simple aspiration, external drainage, and marsupialization of the cyst into the peritoneal cavity by standard surgical technique or laparoscopy [8–12]. Lymphoceles are present in all kidney transplant experiences, and their pathophysiology remains, at the moment, almost unknown. Many contributing factors such as extensive perivascular dissection of iliac vessels, acute rejection episodes, delayed graft function, source of kidney (cadaveric versus living related donor), use of diuretics, and steroid therapy may be involved [13–16]. Recently, retransplantation and adult polycystic disease (ADPKD) in the recipient have also been considered as adjunctive significant risk factors [17, 18]. The two main sources for lymph production are lymphatics close to the iliac vessels and those present in the renal allograft ilium [13, 19, 20]. In the majority of transplant centers, including our own, the external iliac vein and artery or the hypogastric artery are used for vascular anastomoses in renal transplantation. Using this standard technique, wide dissection of perivascular lymphatic vessels is unavoidable in this region. Our opinion is that, in postrenal transplant recipients, the first step in the management of symptomatic lymphoceles should be percutaneous drainage with or without drug instillation. This will stabilize renal function and optimize patients who may require surgery and can, in itself, be curative. However, surgical marsupialization offers a superior one-step definitive treatment of lymphoceles with the lowest recurrence rates.
22.2 Posttransplant Ureteral Obstruction
Stricture of the ureterovesical junction (UVJ) anastomosis, with reported incidence rates of 2–10 %, is the most frequent urologic complication in kidney allograft recipients [21–23]. Significant stricture is a serious complication which can result in kidney failure and permanent damage to the allograft. Open surgery has traditionally been used for correction of the obstruction; however, open procedures are associated with morbidity and delayed convalescence. Development of percutaneous modalities of treatment such as percutaneous nephrostomy (PCN) with low complication rates has altered the approach to ureteral stricture. Percutaneous nephrostomy was first described by Goodwin and colleagues for temporary drainage in cases of hydronephrosis [24]. Nowadays, this procedure is widely used for the treatment of UVJ obstruction in individuals without renal replacement therapy. A number of studies have evaluated PCN in the treatment of ureteral obstruction and urine leakage in kidney transplant patients [25–27]. Ureteral obstruction and leakage are the most common urologic complications encountered in kidney transplant recipients [28–30]. Most series indicate that about 70 % of the ureteral obstructions occur within 3 months of transplantation and 80 % occur at the UVJ site [22, 31, 32]. Prompt diagnosis and early treatment are critical for preventing loss of the allograft and decreasing morbidity and mortality. Ultrasonography and renal scintigraphy can be used as initial diagnostic techniques for assessing the patency and integrity of the renal collecting system. Urinary obstruction manifests by a rising level of serum creatinine, whereas US can easily confirm the diagnosis of hydronephrosis. Diagnosis of the obstruction or leakage can be definitively confirmed using percutaneous anterograde pyelography. Percutaneous approach should be considered as a method of therapy for ureteral stricture regardless of the severity of obstruction [25]. Obstructions that occur soon after transplantation are thought to be due to mechanical causes including blood clots, calculi, edema, and ischemic necrosis, whereas late obstructions are usually the result of local or generalized fibrosis due to ischemia or rejection [24, 30–34]. Fibrosis detected in late obstructions is less likely to resolve with insertion of an intraluminal ureteral stent (Fig. 22.2). As mentioned, PCN is a well-established technique for rapid relief of ureteral obstruction and improvement of the kidney function. However, if this method fails, open surgery will be considered, although it is associated with higher mortality and morbidity rates. Repeated surgery in kidney transplant patient can be extremely difficult and may result in graft loss and/or significant blood loss if not performed by an experienced surgeon.
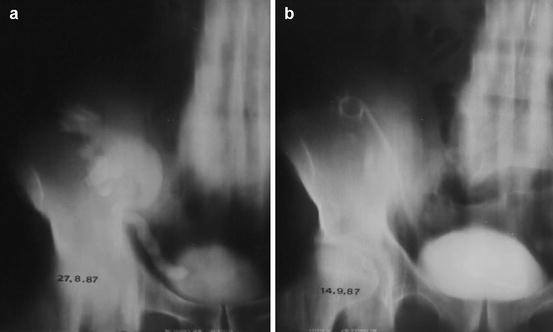

Fig. 22.2
Obstruction of the UVJ with hydronephrosis (a) before treatment and after placement of a ureteral stent (b)
22.3 Urinary Leakage
Leakage is usually the result of ureteral necrosis as a consequence of rejection or vascular insufficiency [33–35] and can be treated by insertion of an indwelling ureteral catheter for 21–60 days without the need for PCN or surgery. Indwelling stents may also be of use as a measure to control urinary leakage and allow stabilization of immunocompromised patients who are too ill to undergo the surgery. Extravasation of urine may occur from the renal pelvis, ureter, or ureteroneocystostomy site due to the surgical technique or ureteral ischemia and necrosis. Urinomas vary in size and usually appear in the first 2 weeks after transplantation between the renal graft and the bladder. Patients with renal leakage may present decreased urine output and manifest pain, tenderness around the graft, discharge from the wound, or even ipsilateral leg swelling and scrotal or labial edema. On US, a urine leak or urinoma appears as an anechoic fluid collection with well-defined borders and lack of septations. Its size increases rapidly, and drainage often needs to be performed with ultrasound guidance to relieve compression and urinary ascites. The higher creatinine level of the fluid compared with its serum concentration differentiates a urine leak from seroma or lymphocele. In addition, urinomas can become infected and eventually form abscesses. Anterograde pyelography is necessary to depict the site of leak and to plan the appropriate intervention (Fig. 22.3). Small urine leaks may be treated with percutaneous nephrostomy and stent placement.
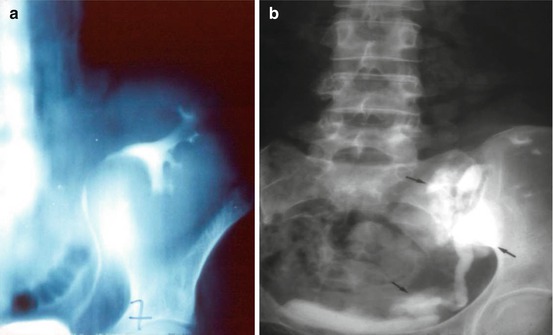

Fig. 22.3
Urinary leakage: normal anterograde pyelography (a) and urinary leakages (b)
22.4 Vascular Complications
Vascular complications occur in fewer than 10 % of renal transplant recipients but are an important cause of graft dysfunction with high associated morbidity and mortality. Despite the fact that magnetic resonance angiography is superior in the diagnosis of vascular complications, color Doppler US, although conventional, remains an excellent noninvasive technique for evaluating vascular pathology [36, 37].
22.5 Renal Artery Stenosis
Transplant artery stenosis is the most common vascular complication (up to 10 %) [38–41]. It usually occurs within the first 3 months [39]. Strictures can affect the iliac artery just proximal to the anastomotic site (atherosclerotic disease in the donor vessel, surgical clamping injury), the anastomosis itself (related to surgical technique), or the proximal renal artery (intimal ischemia). Approximately half of renal artery stenoses can be located adjacent to the anastomosis; moreover, end-to-end anastomoses have a threefold greater risk of stenosis than end-to-side anastomoses [42]. Evaluation for renal artery patency should be performed in several clinical scenarios: (a) severe hypertension refractory to medical therapy, (b) hypertension combined with an audible bruit over the graft, and (c) hypertension associated with unexplained graft dysfunction. Moderate hypertension alone is not a precise marker for renal artery stenosis because up to 65 % of transplant recipients have non-renovascular hypertension. The renal artery is mapped by using color Doppler techniques. The stenotic segments reveal focal color aliasing due to increased flow velocity. Doppler criteria for significant stenosis include the following: (a) velocities greater than 200 cm/s, (b) a velocity gradient between stenotic and prestenotic segments of more than 2:1, and (c) marked distal turbulence (spectral broadening). In the segmental artery branches of the transplant, tardus-parvus waveform abnormalities, decreased resistivity index (RI < 0.56), and loss of early systolic peak may variably be observed [41, 43–45]. Even if these findings exist, when the patient is clinically doing well, only conservative monitoring is performed [43]. When treatment is necessary, percutaneous transluminal angioplasty with or without stent placement is nowadays accepted as the initial treatment of choice [46]. Clinical success in the form of improvement or definite treatment has been reported in 73 % of patients.
22.6 Renal Vein Thrombosis
Renal vein thrombosis is an unusual posttransplant complication; it happens in <5 % of patients within the first postoperative week. Clinical presentation is similar to infarction with abrupt cessation of urinary function, swelling, and tenderness over the graft.
Renal vein thrombosis is more likely to occur following surgical difficulty with the venous anastomosis, episodes of hypovolemia, venous compression by a peritransplant collection, or slow flow secondary to rejection. On US, the kidney may be large and hypoechoic with loss of corticomedullary differentiation. Echogenic material may be seen in the renal vein. Doppler examination shows reduced or no flow in the main renal vein, and there is increased resistance on the arterial conduit, often resulting in reversed diastolic flow in the main renal artery and/or intrarenal arteries [42, 47, 48]. If thrombosis is partial, high RI may be seen [49]. Increased focal venous velocity may also be noted in partial thrombosis, kinking, and extrinsic pressure by fluid collection. However, the combination of this finding with absence of venous flow at the hilum is diagnostic for this condition, and early recognition of this pattern is crucial because the allograft may sometimes be salvaged by prompt thrombectomy.
22.7 Renal Artery Thrombosis
Thrombosis of the main renal artery occurs very rarely (<1 % of cases) in the early postoperative period and usually leads to graft loss. It may result from severe rejection, anastomotic occlusion, arterial kinking, or intimal flap. Patients with renal transplant infarction present with anuria and often with swelling and tenderness over the graft [50].
Color Doppler imaging reveals no arterial and venous flow distal to the thrombus and in the intrarenal vessels. Similar findings can be present at severe rejection. Thrombosis of an accessory renal artery or intrarenal arterial branches will result in segmental infarcts. Although a main artery thrombosis usually results in nephrectomy, there has been some reported success with percutaneous angiographic thrombolytic techniques for treating infarcts. Early diagnosis and treatment are vital for allograft salvage [51].







