Renal Transplantation: Introduction
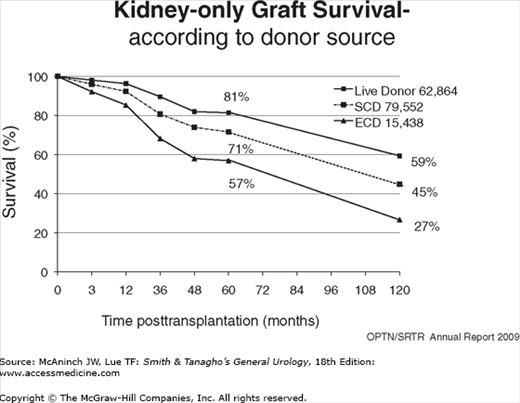
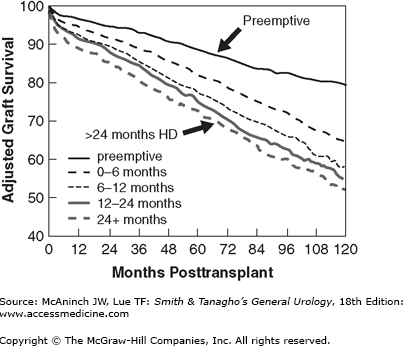
The first successful kidney transplant from a live donor to his identical twin brother was done in 1954. Since then, kidney transplantation has progressed from an experimental procedure to the preferred method of renal replacement therapy around the world. There are three primary reasons for the worldwide acceptance of renal transplantation: (1) transplant recipients enjoy a prolongation of survival compared with dialysis (Wolfe et al, 1999), (2) recipients report an improved quality of life (Joseph et al, 2003), and (3) it is less costly than that of dialysis (USRDS, 2010). At the end of 2010 in the United States, there were about 382,000 patients receiving dialysis therapy, with an incident rate of about 342 per million population, and over 165,000 living with a kidney transplant (USRDS, 2010). In 2010, there were 16,889 kidney transplants performed in the United States, 10,622 from deceased donors and 6277 from live donors (UNOS Web page). However, more than 88,847 patients were actively waiting for a kidney, and the gap between the number waiting and available organs widens every year (Wolfe et al, 2010). Currently, 1- and 5-year kidney graft survival ranges between 89–95% and 66–80%, depending on donor source (Figure 36–1). The major reasons leading to improved outcomes are more potent, yet selective immunosuppression, better surgical techniques, more sensitive tissue typing and crossmatching, and better prophylaxis and treatment of morbid infections. There is also an emerging consensus that preemptive transplantation, immediately prior to the need to dialysis, is advantageous, reducing much morbidity and even mortality (Kasiske et al, 2002).
Selection and Preparation of Recipients
The most frequent diagnoses of renal failure for patients on the transplant waiting list are diabetes, 28% (the fastest growing); all types of glomerulonephritis/focal sclerosis, 25%; hypertension nephrosclerosis, 23%; cystic kidney diseases, 9%; interstitial/pyelonephritis, 5%; urologic diseases, 4%; and unknown causes, 7% (USRDS, 2010). Children <AGE class=reflink xmlns:xlink="http://www.w3.org/1999/xlink" href="#56949813" (NAPRTCS, 2009). Patients older than 65–70 years, the fastest growing recipient group, are commonly transplanted today, as physiological age is considered more important than chronological age (Flechner, 2002). Most patients with end-stage renal disease (ESRD) can be suitable transplant candidates with a few absolute contraindications. These include active infections or cancer, severe vasculopathy from atherosclerosis, and metabolic diseases likely to recur (oxalosis, cystinosis). However, all decisions must be individualized, and patients with a life expectancy of less than 3–5 years probably should be maintained on dialysis. Other factors such as psychosocial status, environment, and ability to follow a complex medical regimen are also important considerations. Prior to transplant, it is important to identify correctable conditions that may increase morbidity and diminish outcomes after the transplant (Flechner, 2002).
It is important that the native urinary tract will function properly after transplant, and an accurate urologic history is essential. Potential recipients without a history of urologic symptoms or prior interventions do not need an extensive evaluation. Upper tract ultrasound and urine cultures usually suffice; some recommend voided cytology. An age appropriate screening prostate specific antigen (PSA) in males. Patients with a history of urologic symptoms (especially hematuria, infections, stones, and incontinence), prior interventions, or a neurogenic bladder should have a full urologic evaluation including upper tract/pelvic imaging, a voiding cystogram, cystoscopy and retrograde studies, cytology, and if indicated a urodynamic study. If patients are dialyzed, upper tract computed tomography (CT) scans with intravenous contrast can be done, while noncontrast images are appropriate prior to dialysis. Gadolinium enhanced magnetic resonance imaging studies remain contraindicated in patients with glomerular filtration rates (GFRs) <60 cc/min, due to the risk of nephrogenic systemic fibrosis (Thomsen, 2009).
Removal of the native kidneys is usually not necessary, done in 10% or fewer patients. Residual urine output and potassium excretion, even if small, as well as production of erythropoietin and vitamin D3 via the retained kidneys are considered beneficial. Medical indications for nephrectomies are rare and include heavy proteinuria (>10 g/day), intractable hypertension (4–5 drugs), and persistent hematuria. Kidneys with chronic hydronephrosis, high-grade reflux, stones, abscesses, filling defects, enhancing masses, complex or very large cysts, etc., that may lead to persistent infections or harbor potential cancers should be removed prior to transplant. In addition, very large polycystic kidneys may need removal for relief of symptoms or size considerations. Potential recipients with acquired renal cystic disease, found in 1/3 to 1/2 of all dialyzed patients, need intervention for contrast enhancing lesions. In most instances, nephrectomy or nephroureterectomy can be done laparoscopically with diminished morbidity (Ghasemian et al, 2005; Ismail et al, 2005). Lesions not removed prior to transplant will need surveillance after the transplant.
It is important to remember that dialyzed patients often have a diminished urine volume, resulting in a small capacity bladder with low compliance. Such bladders will resume normal function, even 25 years later, once urine volume is restored (Serrano et al, 1996). However, small capacity bladders that are fibrotic and scarred from prior surgery, radiation, old TB, and congenital anomalies (posterior urethral valves, meningomyelocele, etc.) will not recover. In these rare cases, often children, the preferred option is a bladder augmentation with bowel (ileum, stomach, colon, or dilated ureter) or a continent neobladder to produce a compliant reservoir with adequate volume (Mendizabal et al, 2004; Nahas et al, 2002; Rigamonti et al, 2005). Bladder augmentation is not without risk, as mucous production, residual urine, and infection often require subsequent intermittent catheterization. If the bladder is absent or destroyed, an ileal conduit can be created for transplantation (Hatch et al, 1993). It is advisable that major surgery is completed and healed prior to transplantation. Experience has taught that operations on a dry urinary tract, that is, bladder neck incisions, urethral stricture repair, and prostatectomy will lead to restricturing and further scarring. Therefore, they should only be done when urine volume is more than a liter per day; or if not, delayed for about 3 months after the transplant. This includes older males who experience progressive prostatic growth absent urine output while on dialysis. These recipients may experience symptoms of prostatism or even urinary retention after the transplant, which will require treatment. During this interval when immunosuppression is reduced and stable engraftment is evolving, recipients are preferably managed by intermittent clean catheterization (Flechner et al, 1983). An indwelling Foley catheter or a suprapubic tube may be necessary if self-cath is not possible. As an alternative, a Spanner stent can be used (Shore et al, 2007).
Active infections are a contraindication to transplantation, which need to be appropriately treated and resolved. This may include surgical drainage of abscesses or removal of a chronic nidus such as infected bone, teeth, maxillary sinus, etc. The urinary tract should be sterile for transplantation. Recurrent urinary tract infections require a full urologic evaluation including upper tract imaging, a voiding cystogram, cystoscopy, and retrograde studies. Recipients with a prior history of tuberculous disease or exposure should receive a year of isoniazid prophylaxis. ESRD patients may be anergic to skin testing.
Herpes family DNA viruses such as cytomegalovirus (CMV), Epstein–Barr virus (EBV), varicella zoster virus (VZV), and herpes simplex virus (HSV) can be transmitted with the donor organ or reactivated from a latent state in the recipient. Therefore, recipients are usually given prophylaxis with a nucleoside inhibitor such as oral valganciclovir for 3–6 months, especially when they are seronegative and the donor is seropositive. Those patients with serologic evidence of prior hepatitis B or C exposure have diminished outcomes, especially if their liver has evidence of cirrhosis. However, those recipients with inactive liver disease who have antibodies to either virus may receive organs from donors that are also positive for either hepatitis B core or hepatitis C antibody (Akalin et al, 2005; Aroldi et al, 2005). A relationship between hepatitis C infection and posttransplant diabetes is also emerging (Bloom and Lake, 2006). Renal failure patients with active and untreated human immunodeficiency virus (HIV) should not be further immunosuppressed by transplant. However, those stable HIV-positive individuals treated with current antiretroviral drug therapy can do well at 1 and 3 years after kidney transplant (Stock et al, 2010).
Active or recurrent malignant disease is an absolute contraindication to renal transplantation. The bulk of evidence suggests that immunosuppressive therapy facilitates the growth of residual cancers. The safe waiting period for transplantation after surgical removal of solid tumors varies and depends on the grade and stage of tumor on presentation and the associated risk of recurrence. Penn (1997) reported that of 1137 neoplasms treated prior to transplantation, the overall recurrence rate was 21%. Fifty-four percent recurred for those waiting only 2 years before transplantation, 33% in those waiting 2–5 years before transplantation, and 13% among those waiting more than 5 years. The highest recurrence rates occurred with breast carcinomas (23%), symptomatic renal carcinomas (27%), sarcomas (29%), bladder carcinomas (29%), nonmelanoma skin cancers (53%), and multiple myeloma (67%). Therefore, with some exceptions, a minimum waiting period of 2 years for cancers with a favorable prognosis is desirable. A waiting period of 5 years is desirable for lymphomas, most carcinomas of the breast, colon, or for large (>5 cm) symptomatic renal carcinomas. No waiting period is necessary for incidentally discovered small renal carcinomas, in situ carcinomas, and possibly tiny focal neoplasms. More recently, it has been suggested that rather than using fixed waiting times, it is more logical to use cancer recurrence nomograms to establish risk. For example, such nomograms have been well established for localized prostate cancer (Kattan, 2008).
Patients with certain metabolic diseases affecting the kidney such as Fabry’s disease, hemolytic uremic syndrome, vasculitis, systemic lupus erythematosus, amyloidosis, etc., as well as various forms of glomerulonephritis and focal sclerosis may experience recurrence, and patients should be counseled regarding this possibility (Couser, 2005). Those with severe metabolic stone disease that resulted in kidney loss may experience recurrent stones and a poor outcome. A combined hepatic and kidney transplant is now commonly recommended for primary hyperoxaluria (Jamieson, 2005) and less so for cystinosis (Rogers et al, 2001).
Cardiovascular disease represents the leading cause of death after kidney transplantation, and is ubiquitous among renal failure patients, especially diabetics and those older than 50 years. Potential recipients should be thoroughly screened and have symptomatic lesions corrected prior to transplant, since those with ESRD are at high risk for ischemic events (Pilmore, 2006). Since many dialysis patients are sedentary, already have abnormal EKG patterns and diabetics may not experience angina with exertion, provocative stress tests are necessary. However, patients should reach their target heart rate for these tests to have an accurate predictive value, but may be limited in long-standing diabetics (Welsh et al, 2011). If any uncertainty exists, the gold standard remains coronary angiography. In an analysis of dialysis patients undergoing coronary revascularization, Herzog et al (2002) found that although the in-hospital mortality was greatest for those undergoing coronary artery bypass graft (CABG) (8.6%) compared with patients having stents (4.1%) or percutaneous transluminal coronary angioplasty (PTCA) (6.4%), the 2-year patient all-cause survival was significantly superior for those after CABG (56.4%) than after stenting (48.4%) or PTCA (48.2%). Patients with a history of strokes or transient ischemic attacks should be screened with a carotid ultrasound and receive neurology clearance. Those with adult polycystic kidney disease need a brain magnetic resonance angiogram to screen for aneurysms. Peripheral vascular disease is common in renal failure, especially diabetics, and pulse volume recordings (PVRs) of the lower extremities can be helpful. A pelvic CT scan without contrast can determine the degree of calcification of the target pelvic vessels and aid in kidney placement. Active claudication, femoral bruits, or diminished pulses demands a complete vascular surgical assessment.
Patients with ESRD often have a history of gastrointestinal problems such as peptic ulcer disease, gastroesophageal reflux, cholecystitis, pancreatitis, inflammatory bowel disease, diverticulosis, chronic diarrhea or constipation, or hemorrhoids. If present, these should be evaluated and resolved prior to transplant. Upper or lower gastrointestinal endoscopy, and/or contrast imaging of the bowel, may be required. Routine cholecystectomy for asymptomatic cholelithiases is no longer advised (Jackson et al, 2005).
In North America, obesity is affecting a greater number of patients with renal failure each year. Numerous reports have identified obesity (body mass index [BMI] >30 kg/m2) and morbid obesity (BMI >35 kg/m2) as an independent risk factor for increased cardiovascular mortality, decreased graft survival, delayed graft function (DGF), wound complications, posttransplant diabetes, proteinuria, and prolonged hospitalization (Armstrong et al, 2005; Gore et al, 2006; Modlin et al, 1997). Weight reduction to lower the morbidly obese range is desirable and may require bariatric surgery in extreme circumstances (Modanlou et al, 2009).
Tobacco smoking is particularly deleterious for transplant recipients, and patients need to stop prior to transplantation. Smoking accelerates the progression of atherosclerotic cardiovascular disease and is nephrotoxic to the kidney resulting in proteinuria (Orth, 2004; Tozawa et al, 2002).
The use of intentional third-party blood transfusions to modulate the immune system is no longer done. In fact, transfusions are generally avoided to prevent the possibility of disease transmission (hepatitis, HIV, etc.) and recipient sensitization to human leukocyte antigen (HLA) phenotypes that may diminish the chance of a negative cross match with a potential donor. Anemia of renal failure is effectively treated with recombinant erythropoietin for most patients (Cody et al, 2005).
After a failed transplant, immunosuppression is weaned off and the patient returns to dialysis. If graft loss occurs after a year, it is usually not necessary to remove the failed graft, as a new kidney can be placed on the contralateral side. In a few cases, when graft failure is early or is due resistant rejection, the kidney tissue may undergo necrosis and the graft needs to be removed. Indications for allograft nephrectomy include fevers, graft tenderness, gross hematuria, malaise, infection, and uncontrolled hypertension. The subcapsular allograft nephrectomy is the safest approach to prevent iliac vessel injury. This technique can be augmented with presurgical angioinfarction of the allograft to reduce surgical blood loss and the need for transfusion (Westesson et al, 2011).
Selection of Donors
Living kidney donation provides better patient and allograft survival when compared with deceased-donor transplantation, especially when the live-donor transplant is performed before the onset of dialysis (Figures 36–1 and 36–2) (Meier-Kriesche et al, 2002). Living donation rates vary worldwide, but in many Western countries, Asia, and the Middle East, it has become the predominant form of kidney transplantation. In the United States, the annual number of live kidney donors has surpassed the number of deceased donors since 2001, although the absolute number of transplants from deceased donors still outnumbers those from living donors (Klein et al, 2010). Living donors are most often directed; they have an established relationship with the intended recipient. Based on tissue typing disparities (HLA mismatches), an immunologic hierarchy can be established for the best “match” (Table 36–1). The advantages for identical twins and HLA-identical siblings are quite significant, while all other live-donor combinations are similar and provide significant advantages to the deceased donor. More than 30% of live donors are genetically unrelated to their recipient, and represent the fastest growing category of donors. These living unrelated donors (LURDs) (Figure 36–3) come from a spouse, a friend, or even someone anonymous to their recipient (nondirected). The ethical underpinning of this evolving practice is the excellent survival achieved by LURD transplantation, which is similar to the survival of a kidney from a parent or child, from a haploidentical sibling, or from a completely mismatched related donor (Cecka, 2004). These observations have influenced decisions regarding the suitability of live donors who are spouses, friends of the recipients, or anonymous. Today, there is little concern about the degree of HLA match if the ABO blood type and T-cell cross match are compatible. The gender of the living donor in the United States is more frequently female, constituting 60% of the live-donor population (Axelrod et al, 2010). This pattern is similar to what has been observed worldwide, with more male recipients undergoing live-donor transplantation. However, among similarly matched groups, kidneys that provide a greater “nephron dose” (anatomically ideal, young, large, male donors) are often preferred.
The extreme shortage of kidneys to meet the demand of waiting recipients coupled with the success of LURD kidney transplantation has opened up creative ways to expand the pool of live donors. In particular, there are individuals who wish to be anonymous donors, that is, “nondirected or altruistic donor.” However, in the United States, living-donor exchanges must adhere to section 301 of the National Organ Transplant Act of 1984 (NOTA), which states, “It shall be unlawful for any person to knowingly acquire, receive, or otherwise transfer any human organ for valuable consideration for use in human transplantation.” Valuable consideration according to this Act has traditionally been considered to be monetary transfer or a transfer of valuable property between the donor and the recipient. The donation of an organ is properly considered to be a legal gift. With these constraints, any person who is competent, willing to donate, free of coercion, and found to be medically and psychosocially suitable may be a live kidney donor (Adams et al, 2002). Three protocols of nondirected living donation have been developed to accommodate such donors: (1) a live-donor paired exchange, (2) a live-donor/deceased-donor exchange, and (3) altruistic donation.
This approach involves exchanging donors who are ABO or cross match incompatible with their intended recipients so that each donates a kidney to a compatible recipient (Delmonico, 2004). The exchange derives the benefit of live donation but avoids the risk of incompatibility; several computer algorithms have been modeled to execute the exchange (Montgomery et al, 2005). The best example is two families, one with an A donor to a B recipient and the second with B donor to an A recipient. Swapping donors solves the dilemma. Live-donor exchange procedures have been performed worldwide and are best performed with large sharing pools (Segev et al, 2005). The popularity of paired kidney donor exchanges has been bolstered by the demonstrated safety of shipping live donor kidneys between centers.
Another system of exchange of donors was devised by centers in UNOS region 1, by permitting the live donor to be used by another compatible individual on the waiting list in “exchange” for the next blood type compatible deceased donor in the region, for the live donor’s recipient. With this method, two patients will be transplanted instead of only one, although some fine-tuning of donor organ quality and age is necessary (Delmonico, 2004).
Altruistic kidney donation (to a complete stranger) is developmental in several centers and must be approached with utmost sensitivity, especially today when organ exchanges are advertised on the Internet. Participating centers usually offer the kidney to the highest wait listed patient at their center after a match run. However, the use of such altruistic donors may aid the formation of live donor “chains” (Rees et al, 2009). The motives of the nondirected donor should be established with care to avoid a prospective donor’s intention of remedying a psychological disorder via donation. Many who inquire about altruistic donation have only a limited understanding of these issues, and upon learning these basic realities, about 60% withdraw from the process (Jacobs et al, 2004).
From its inception, the removal of a kidney from a healthy individual to benefit another has been problematic. The practice is based upon the belief that the removal of one kidney is safe and does not diminish survival or significantly harm long-term kidney function. This notion derives from follow-up of patients up to 45 years after nephrectomy for trauma (Narkun-Burgess et al, 1993). Ibrahim et al (2009) reported the follow-up of 3698 kidney donors between 1963 and 2007 and found that survival and the risk of ESRD appeared to be similar to those in the general population. They found that ESRD developed in 11 donors, a rate of 180 cases per million per year versus 268 cases per million in the general population. At a mean (±SD) of 12.2 ± 9.2 years after donation, 85.5% of a subgroup of 255 donors had a measured GFR >60 mL/min/1.73 m2, 32.1% had hypertension, and 12.7% had albuminuria. Older age and higher BMI, but not a longer time since donation, were associated with both a GFR <60 mL/min and hypertension. In addition, a recent analysis of 4650 donors between 1987 and 2007 in which 76.3% were white, 13.1% black, 8.2% Hispanic, and 2.4% other found that both black and Hispanic donors had an increased risk of hypertension, diabetes mellitus requiring drug therapy, and chronic kidney disease (Lentine et al, 2010). ESRD developed more frequently in blacks, but it was <1% for the donor population studied. These recent studies are in line with prior reports that unilateral nephrectomy caused an average decrease of 30% in the GFR that tended to improve with each 10 years of follow-up (average increase 1.4 mL/min per decade); a small, progressive increase in proteinuria (average 76 mg/decade); and variable effects on hypertension (Kasiske et al, 1995). Thus, the published evidence indicates that there is little long-term medical risk to a healthy donor after unilateral nephrectomy. Nevertheless, Ellison et al (2002) identified 56 live kidney donors who were subsequently listed for a kidney transplant. The rate of ESRD in donors was calculated to be 0.04%, comparable with the rate (0.03%) in the general population. The renal diagnosis in these patients was hypertension, focal sclerosis, chronic glomerulonephritis, familial nephropathy, diabetes, and other. Recently, some have advocated the use of donors with isolated medical abnormalities such as hypertension, obesity, dyslipidemia, or stones, which may not result in the safety profiles previously reported. As of 2011, prior living donors get preference for deceased donor kidneys should they develop ESRD (UNOS Web site).
The imbalance between the supply of brain dead deceased donors and the growing demand for kidneys has created many innovative uses of organs that were excluded in the past. These generally include kidneys from donors older than 60 years; the presence of systemic disease such as atherosclerosis, hypertension, or early diabetes; donors with cardiac arrest or significant hypotension; and some with prior exposure to virus and/or infections that have resolved (Ismail and Flechner, 2006). While kidneys that are severely traumatized or come from donors with active cancer, sepsis, or HIV–AIDS are excluded, a number of donor organs with extended criteria that convey about a 10% worse overall graft survival have been incorporated into the donor pool. To maximize kidney usage, the following categories have been developed.
Most individuals that meet the criteria for brain death from age 5 to 60 years with normal kidney function and no history of systemic or infectious disease.
Kidneys from brain dead donors with 1.7 times relative risk of graft failure. These criteria were developed from a consensus conference that analyzed registry survival data (Rosengard et al, 2002). These include any donor older than 60 years or older than 50 years with a history of hypertension, cerebrovascular accident (CVA) death, or creatinine >1.5 mg/dL (Table 36–2). Informed consent of the recipient is requested to receive an expanded criteria donor (ECD) kidney.
When a potential donor does not meet brain death criteria but has an irretrievable head injury, viable organs for transplant can be procured after a controlled cardiac arrest. Such kidneys experience a greater incidence of DGF, but long-term function is comparable with standard donor kidneys (Rudich et al, 2002).
At the extremes of life, one kidney may not be sufficient to deliver an adequate GFR (nephron dose) to an adult recipient. In these instances, using both kidneys from a single donor can overcome these limitations.
Kidneys from donors younger than 5 years (often <6 cm in length) have a historically higher failure rate from technical problems and develop hyperfiltration injury (proteinuria) when transplanted into adults (Bresnahan et al, 2001). Both kidneys can be transplanted en bloc, attached to the donor aorta and vena cava, in a more reliable fashion (Hobart et al, 1998). Such kidneys will grow to adult size in 6–12 months.
When kidneys have extremely unfavorable risk factors for graft success due to insufficient nephron mass, both may provide for successful outcome (Bunnapradist et al, 2003). Such adult dual transplants can be placed in either iliac fossa or preferably on the same side through one incision (Flechner, 2008). The criteria established for dual kidney allocation appear in Table 36–3. This approach utilizes kidneys that in the past were often discarded.
|
Once removed, kidneys are flushed to remove blood and stored in a hyperosmolar, hyperkalemic, and hyponatremic solution at (4–10°C) to minimize ischemic injury (cellular swelling). This is usually sufficient for up to 24 hours of preservation although longer cold ischemic times (up to 40 hours) have been reported, but result in higher rates of DGF. A commercial storage solution from the University of Wisconsin (UW) is frequently used, which contains inert substrates such as lactobionate, raffinose, hydroxyethyl starch, and adenosine as an energy substrate. Recently, a less viscous alternative histidine-tryptophan-ketoglutarate (HTK) solution has been shown to yield similar results with cold ischemia times <24 and >24 hours (Agarwal et al, 2006).
Hypothermic pulsatile perfusion is an alternative method of preservation, which takes advantage of a continuous pulsatile flow through the graft. Some feel that such hydrodistention is therapeutic in dilating the ischemic renal microcirculation and permits the delivery of vasodilator drugs (ie, verapamil, beta-blockers). It also permits measurement of flow, pulse pressure, and resistance through the graft, which is an accurate method to determine viability of the kidney (Schold et al, 2005). Pulsatile perfusion is more costly and requires investment in a preservation unit (Waters Co, Rochester, MN) and a technologist, but has been gaining popularity due to the increasing number of ECD and donation after cardiac death (DCD) donors that are considered for transplant (Matsuoka et al, 2009; Shah et al, 2008).
The major histocompatibility complex (MHC) describes a region of genes located on chromosome 6 in man, which encode proteins that are responsible for the rejection of tissue between different species or members of the same species (Flechner et al, 2011). The cell surface MHC markers are called human leukocyte antigens (HLAs), because they were first identified on white blood cells. There are two major types of HLA antigens termed class I and class II. Virtually all nucleated cells express HLA class I antigens, while class II antigens are primarily found on B cells, monocytes, macrophages, and antigen-presenting cells. Each individual inherits two serologically defined class I (named A and B) and one class II (named Dr) antigen from each parent, so six HLA antigens constitute an individual’s tissue type. One set of HLA A, B, and Dr antigens inherited on one chromosome from a parent is called a haplotype so that HLA-identical siblings have inherited both haplotypes. The HLA molecules are polymorphic (over 170 defined), so it is very unusual if two unrelated individuals have the same tissue type of six HLA antigens. HLA antigens not shared between two individual will generate an immune response. Therefore, the term HLA matching describes the number of shared antigens. One can generate a hierarchical rating of genetic HLA similarities, which roughly correlate to the risk for rejection and eventual kidney transplant outcomes ranging from identical twins to deceased donor (DD) (Table 36–1). In clinical practice, the impact of HLA on graft survival is small in the initial years but may play an important role after 5–10 years. No doubt other factors affect survival: especially donor organ quality (age, function, size, etc) as well as recipient age and comorbidities. However, at present, six Ag-matched (or zero HLA mismatched) deceased donor kidneys are shared nationally due to the beneficial effect on immunological outcomes. In addition, HLA antigen matches also play a role in the algorithm for distribution of deceased donor kidneys with more points assigned for better matches.
Preformed circulating anti-HLA antibodies against the specific phenotype of the donor will lead to acute (if not hyperacute) rejection. Such antibodies (usually IgG) are detected by crossmatching the sera of the recipient with lymphocytes of the donor and adding complement. Such complement-dependent cytotoxicity (CDC) will kill the donor cells and is indicative of deleterious clinical outcome. A similar, yet more sensitive, test has been developed using flow cytometry to identify the presence of anti-HLA antibodies bound to the surface of donor lymphocytes. A crossmatch against both donor T and B lymphocytes is performed within 24 hours of surgery, and transplants are not done if these antibodies are strongly present. In addition, the ABO system will trigger CDC against the mismatched blood group antigens (glycoproteins) present on many tissues. Therefore, transplants are usually done only between ABO compatible individuals. In the last few years, more transplants have been done with weak ABO incompatibilities (low anti-A or anti-B titers) with good outcomes (Montgomery et al, 2009).
At monthly intervals waiting, patients have their serum screened for the presence of anti-HLA antibodies against a panel of HLA phenotypes (lymphocytes) that represent the general population. The result is reported as a percentage of the total referred to as percent reactive antibody (PRA). Those with high titers (>50%) of anti-HLA antibody against the broad population are said to be sensitized and will find it very hard to find a cross-match negative donor. Sensitized patients waiting for an organ depend on better HLA matches to find a cross-match negative donor (McCune et al, 2002). Sensitization to HLA can occur from prior blood transfusions, viral infections, pregnancy, or previous transplants.
The development of de novo donor-specific or nondonor-specific anti-HLA antibodies after the transplant has a deleterious effect on outcomes. Both a greater frequency of acute and chronic rejection as well as lower graft survival have been reported among those patient with these antibodies detected by flow cytometry (El Fettouh et al, 2001; Hourmant et al, 2005). The presence of these antibodies may identify those recipients that need more rather than less immunosuppression. The recent introduction of solid phase technology in which specific HLA antigens bound to synthetic beads can be used as a target to screen sera for the presence of HLA antibodies has expanded our ability to monitor recipients before and after transplant (Lefaucheur et al, 2010).
Removal of a kidney for transplant depends upon minimizing both surgical injury and warm ischemia, which will hasten the recovery of function in the recipient. It is best to ensure a brisk diuresis in the donor before the kidney is removed, which can be enhanced by the use of volume expansion with saline and albumin, osmotic diuretics (mannitol), and loop diuretics (furosemide) in order to maximize immediate graft function in the recipient. Minimal dissection of the renal hilum is preferred (Flechner et al, 2008).
All donors should be evaluated both medically and surgically to ensure donor safety. An outline of the usual donor evaluation is shown in Table 36–4
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree