Renal Mesenchymal and Mixed Mesenchymal-Epithelial Tumors
Ying-Bei Chen
ANGIOMYOLIPOMA
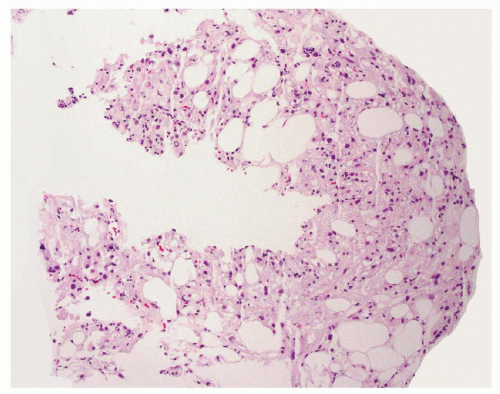
The classical renal angiomyolipoma (AML) is a benign mesenchymal tumor comprising variable amount of fat, smooth muscle cells, and abnormal thick-walled vessels. These tumors are believed to arise from perivascular epithelioid cell (PEC), similar to other PEC-related tumors such as lymphangioleiomyomatosis, clear cell “sugar” tumors of lung, and PEComas of other organ sites. The morphologic variants of AML described in the literature have included epithelioid, fat-predominant, smooth muscle-dominant, oncocytic, AML with epithelial cysts (AMLEC), and sclerosing types. The contemporary imaging technologies can readily recognize AMLs when they contain a significant amount of fat. However, AMLs with little fat or other uncommon features closely mimic renal epithelial neoplasms on radiology studies, which often lead to a surgical resection or renal needle core biopsy.
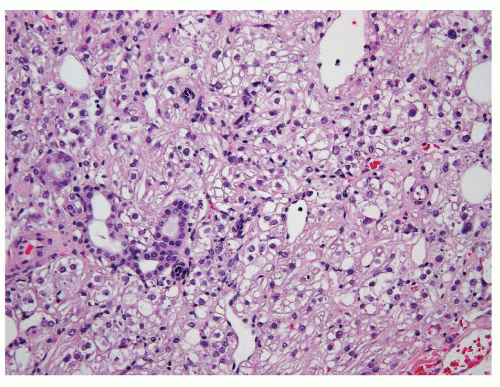
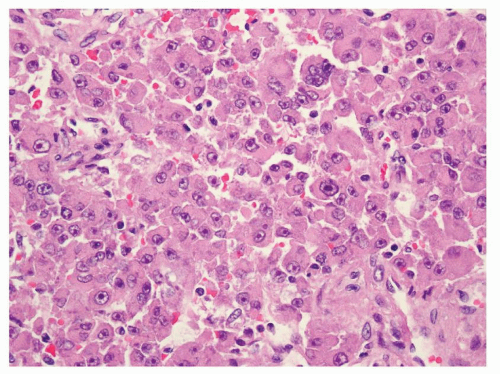
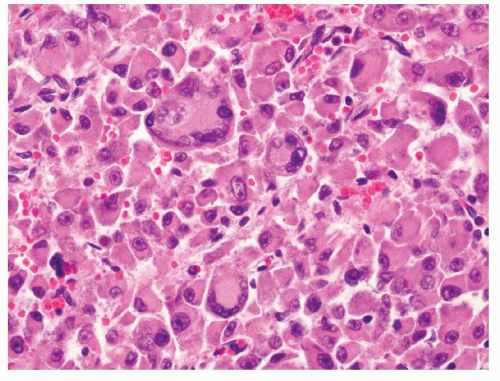
Because of the limited sampling, the classical triphasic appearance of AMLs may be lacking in core biopsies. Thus, any intermingled presence of scattered mature adipose tissue or rare adipocytes together with fascicles of spindle cells (Fig. 6.1) or sheets of epithelioid cells (Fig. 6.2) should raise a suspicion for this diagnosis. Moreover, it is not uncommon that adipocytes may be entirely absent in some AMLs such as those with predominantly leiomyomatous differentiation, epithelioid AMLs, or AMLECs. One of the main challenges of diagnosing AML in core biopsies is to recognize the broad range of histologic features that the spindle or epithelioid cells display in these tumors. Often, the cells have eosinophilic to somewhat clear cytoplasm, with a fibrillary and/or granular quality (Fig. 6.3). Others may be difficult to distinguish from typical smooth muscle cells (Fig. 6.4). Epithelioid AMLs are often composed of sheets of large round or polygonal cells with abundant eosinophilic, granular or vacuolated cytoplasm, and enlarged vesicular nuclei that often show prominent nucleoli. In core biopsies, this appearance closely mimics malignant epithelial cells with marked
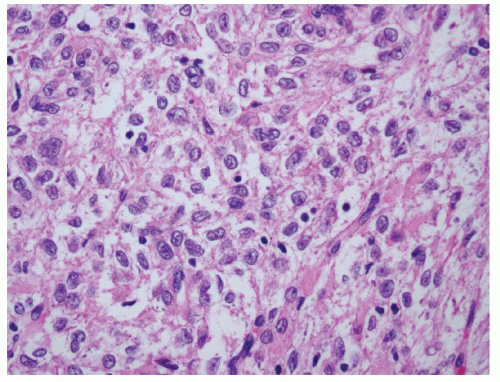
cytologic atypia and nuclear pleomorphism (Fig. 6.5). Multinucleation and ganglion-like cells may also be present (Fig. 6.6). Mitoses are common, rare atypical mitoses may also be observed. On the other extreme of the spectrum, a predominantly lipomatous AML often only shows focal spindle cell proliferation (Fig. 6.7). Immunohistochemistry (IHC) is very helpful for establishing the definitive diagnosis in AMLs with uncommon features.
cytologic atypia and nuclear pleomorphism (Fig. 6.5). Multinucleation and ganglion-like cells may also be present (Fig. 6.6). Mitoses are common, rare atypical mitoses may also be observed. On the other extreme of the spectrum, a predominantly lipomatous AML often only shows focal spindle cell proliferation (Fig. 6.7). Immunohistochemistry (IHC) is very helpful for establishing the definitive diagnosis in AMLs with uncommon features.
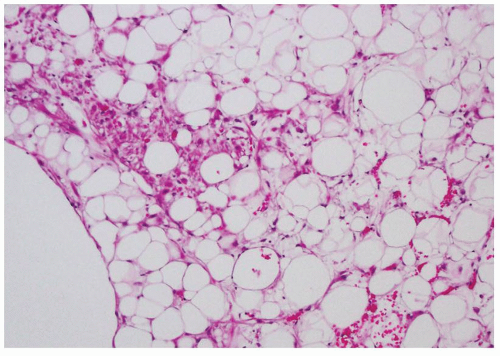 FIGURE 6.7 Focal epithelioid and spindle cell proliferation in a fat-predominant AML. These cells show immunoreactivity to HMB-45. |
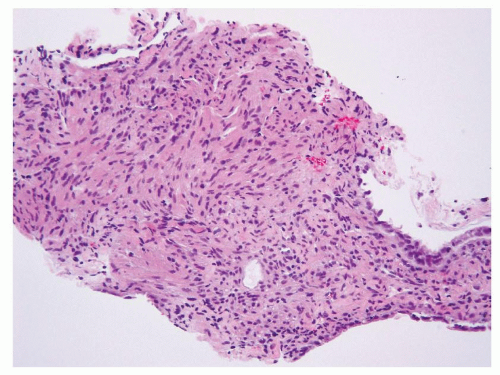
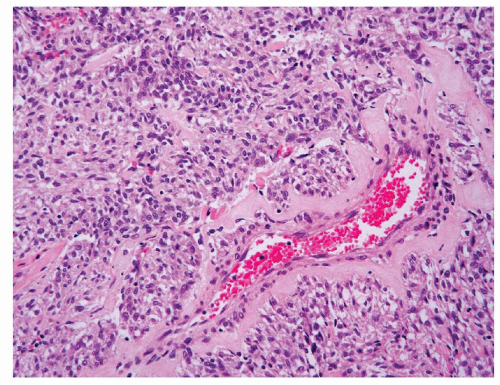
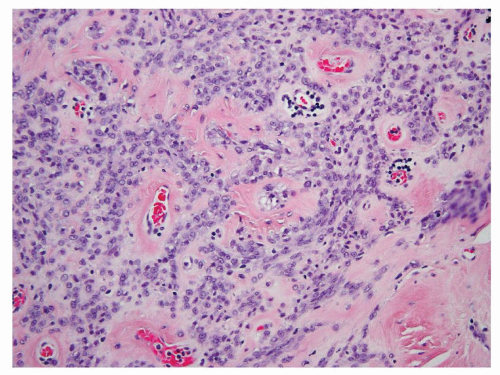
Dysmorphic vessels such as those with thickened and hyalinized wall may occasionally present in a core biopsy and serve as a very helpful clue. Smooth muscle cells often appear to originate from such vessels (Fig. 6.8). In the rare variant of sclerosing AML, cords or sheets of cytologically uniform and bland epithelioid cells are often arranged around these vessels in a densely sclerotic stroma (Fig. 6.9).
AMLEC is another rare variant of AML in which there are epithelial cysts lined by cuboidal to “hobnailed” cells (1). Often a “cambium-like” subepithelial layer of cellular, müllerian-like stroma with prominent admixed chronic inflammation is found underneath the epithelium, whereas the more typical AML with associated dysmorphic blood vessels is further exterior to these components (Fig. 6.10). It remains unclear whether the epithelial cysts in AMLEC represent entrapped renal tubules (2) or are an integral component of the tumor.
Although the vast majority of AMLs are considered benign tumors, some epithelioid AMLs have been reported to develop metastasis or recurrence. The overall incidence of metastasis among all cases of epithelioid AMLs is somewhat difficult to determine because of their rarity and the variable criteria used to define this variant. The reported rates have varied from near 50% in some consultation cohorts to about 5% in other primary resection cohorts (3,4,5,6). Some reported series have used at least 50% to 80% of epithelioid cells as the requirement to designate as epithelioid AML, and the reported adverse prognostic pathologic indicators include pure epithelioid histology, large tumor size (>7 cm), perirenal tumor extension, frequent mitoses (>2/10 high-power fields) and necrosis, etc.
Ancillary Studies
AMLs often coexpress melanocytic markers such as HMB-45, Melan-A (MART-1), MiTF, tyrosinase, and smooth muscle markers including smooth muscle actin, caldesmon and calponin, etc. Recently, cathepsin K has been found to be a very sensitive marker for AML (7). AMLs are usually negative
for epithelial markers (cytokeratin, EMA/MUC1), except in epithelial cysts seen in AMLEC. However, the expression of melanocytic markers may be limited to one of the markers in a given tumor and the immunoreactivity can be focal and/or weak (Fig. 6.11). The subepithelial stroma in AMLEC is often positive for estrogen receptor (ER), progesterone receptor (PR), and CD10, whereas the epithelial cysts express PAX8 (eFig. 6.1).
for epithelial markers (cytokeratin, EMA/MUC1), except in epithelial cysts seen in AMLEC. However, the expression of melanocytic markers may be limited to one of the markers in a given tumor and the immunoreactivity can be focal and/or weak (Fig. 6.11). The subepithelial stroma in AMLEC is often positive for estrogen receptor (ER), progesterone receptor (PR), and CD10, whereas the epithelial cysts express PAX8 (eFig. 6.1).
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree