RENAL HANDLING OF CALCIUM, PHOSPHATE, AND MAGNESIUM
CALCIUM
More than 98% of total body calcium is in bones, whereas the remainder is located in intracellular and extracellular fluid. Normal plasma concentrations, which range from 8.8 to 10.3 mg/dL, are maintained by the actions of PTH, 1,25-hydroxyvitamin D, and calcitonin on bones, the gastrointestinal tract, and the kidneys.
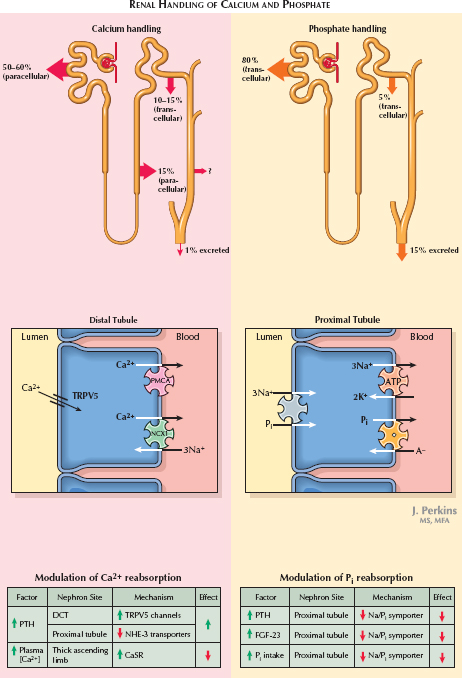
About half of the extracellular calcium load is in an active, ionized form, whereas the remainder complexes with albumin and other anions. The ionized calcium is freely filtered at the glomerulus, and normally almost all of it is reabsorbed.
In the proximal tubule, 50% to 60% of the filtered load is reabsorbed along a paracellular route. A chemical gradient is established as sodium and water are reabsorbed, concentrating calcium in the tubular fluid. Meanwhile, an electrical gradient is established by the paracellular reabsorption of chloride, which leaves a positive charge in the lumen. Specialized tight junction proteins, such as claudin-2, may form a cation-specific paracellular pathway.
In the thick ascending limb, 15% of the filtered load is reabsorbed along a paracellular route. An electrical gradient, formed secondary to K+ recycling, drives this process. Claudin-16, another tight junction protein, is an important component of this paracellular pathway, and mutations are associated with familial hypomagnesemia with hypocalciuria.
In the distal convoluted and connecting tubules, 10% to 15% of the filtered load is reabsorbed along a transcellular route. Calcium crosses the apical membrane through TRPV5 channels, binds to calbindin, then exits the basolateral membrane on the NCX1 Na+/Ca2+ exchanger and, to a lesser degree, a Ca2+ ATPase (PMCA).
The collecting duct makes an unknown, but likely minor, contribution to calcium reabsorption.
Hypocalcemia triggers release of PTH, which has numerous effects on renal function. In the proximal tubule, it inhibits the NHE-3 Na+/H+ exchanger, reducing the gradient for paracellular calcium reabsorption. (This seemingly paradoxical effect allows PTH to increase phosphate excretion, as discussed later.) In the distal nephron, however, it up-regulates the apical TRPV5 calcium channel, causing a net increase in calcium reabsorption. Meanwhile, hypercalcemia both suppresses PTH release and directly inhibits calcium reabsorption. In the thick ascending limb, for example, the increased load of reabsorbed calcium activates a basolateral calcium-sensing receptor (CaSR), which then inhibits NKCC2 transporters and ROM-K channels, reducing the electrical gradient for calcium reabsorption.
Finally, acidosis inhibits the TRPV5 calcium channel, whereas alkalosis has the opposite effect.
PHOSPHATE
About 85% of total body phosphate is stored in bones, 14% in soft tissues, and 1% in extracellular fluid. Normal plasma concentrations, which range from 3 to 4.5 mg/dL, are maintained by the actions of PTH, 1,25-hydroxyvitamin D, and phosphatonins on the parathyroid glands, bones, gastrointestinal tract, and kidneys.
About 90% of plasma phosphate is unbound and freely filtered at the glomerulus. About 85% of the filtered load is normally reabsorbed.
In the proximal tubule, 80% of the filtered load is reabsorbed along a transcellular route. Phosphate crosses the apical membrane on Na+/Pi symporters. The pathway of basolateral exit is poorly understood but may involve a phosphate/anion exchanger.
In the distal convoluted tubule and connecting tubule, 5% of the filtered load is reabsorbed along a transcellular route that remains poorly understood.
Hyperphosphatemia promotes release of PTH, which down-regulates Na+/Pi symporters and basolateral Na+/K+ ATPases in the proximal tubule. As a result, phosphate reabsorption is suppressed. Additionally, hyperphosphatemia causes the release of FGF-23 from osteocytes and osteoblasts in bone, which causes decreased Na+/Pi expression. Finally, increased dietary phosphate intake appears to directly down-regulate Na+/Pi transport through a PTH-independent mechanism. Hypophosphatemia, meanwhile, causes the opposite effects.
MAGNESIUM
About half of total body magnesium is in bone, and nearly all of the remainder is in intracellular fluid. Only 1% is in the extracellular space, with normal plasma concentrations ranging from 1.8 to 2.3 mg/dL. About 80% of the extracellular load is unbound to proteins and freely filtered at the glomerulus. About 95% to 98% of the filtered load is normally reabsorbed. 20% is reabsorbed in the proximal tubule via an unknown, likely passive, mechanism. Another 60% to 70% is reabsorbed in the thick ascending limb through a paracellular route, driven by the electrical gradient resulting from K+ recycling. Claudin 16 is thought to form the pore for paracellular magnesium reabsorption. Finally, 5% to 10% of the filtered load is reabsorbed in the distal nephron through an apical Mg2+ channel known as TRPM6. The pathway for basolateral exit is not known.
< div class='tao-gold-member'>




