Renal Cell Carcinoma: Uncommon Types
Satish K. Tickoo
Ying-Bei Chen
Most of the tumors described in this chapter are relatively rare and, in theory, may raise the apprehension of being missed or misdiagnosed on needle core biopsies. However, as in resection specimens, in real practice, a vast majority of them can be diagnosed on needle core biopsies based on their characteristic morphologic features. At the same time, given the relative lack of experience for most pathologists with these tumors, it may be prudent to use ancillary techniques—in particular immunohistochemistry—more liberally until greater experience with these is garnered.
MULTILOCULAR CYSTIC RENAL CELL NEOPLASM OF LOW MALIGNANT POTENTIAL/MULTILOCULAR CYSTIC RENAL CELL CARCINOMA
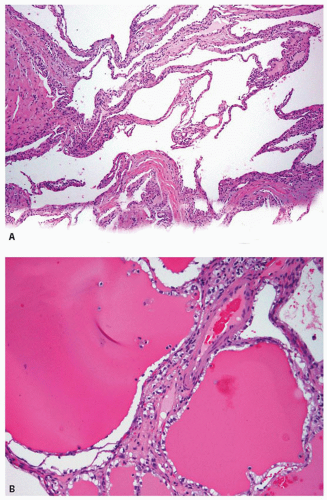
Multilocular cystic renal cell carcinoma represents a rare subset of clear cell renal cell carcinoma (RCC) (1,2,3,4,5), as it shows similar molecular alterations as clear cell RCC (1,6). However, the follow-up information available on these tumors has shown no recurrence or metastases (2,3,4,5). Therefore, it is suggested that these lesions be redesignated as multilocular cystic renal cell neoplasms of low malignant potential (4,5). By definition, it is a tumor composed entirely of numerous cysts, with the septa containing dispersed clusters of clear cells with low nuclear grade. Presence of any solid areas or expansile masses in the tumor excludes it from the category of multilocular cystic RCC. It is, therefore, apparent that even when only multilocular cystic component of a tumor with clear cell lining is seen on a needle core biopsy, one cannot diagnose it as multilocular cystic RCC with certainty. In such instances, one can only raise the possibility of multilocular cystic RCC and suggest correlation with radiologic findings.
Microscopically, the thin fibrous septae are lined by one to multiple layers of cells with clear cytoplasm and low-grade nuclei; although the epithelial lining may be absent in many areas (Fig. 4.1A). Focal papillary tufting of the lining may also be seen. Foamy macrophages may also line the cyst wall. Small clusters of tumor cells are present within the fibrous septa
(Fig. 4.1B) or in the adjacent pseudocapsule, but no expansile masses that alter the shape of the septae are present. In biopsy specimens, depending on the size of the cysts, only collapsed cyst walls with sprinkling of the clear cells in the walls may be noted (Fig. 4.1A).
(Fig. 4.1B) or in the adjacent pseudocapsule, but no expansile masses that alter the shape of the septae are present. In biopsy specimens, depending on the size of the cysts, only collapsed cyst walls with sprinkling of the clear cells in the walls may be noted (Fig. 4.1A).
Ancillary Studies
Immunohistochemical support may be needed particularly in biopsies with collapsed cyst walls and scant lining cells. Carbonic anhydrase IX (CA-IX) and CK7 diffusely highlight the lining cells supporting the morphologic impression (7) and may also help in differentiation from cystic nephroma (see following discussion).
Differential Diagnosis
In addition to clear cell RCC with prominent cystic component, the differential diagnosis includes cystic nephroma and benign multilocular cyst of the kidney. Close attention to the epithelial lining and the fibrous cyst wall will lead to the correct diagnosis. Cystic nephromas are usually lined by a single layer of flattened or hobnailed epithelium, which only rarely shows clear cytoplasm. The fibrous stroma is often cellular, resembling ovarian stroma. The septa may contain small tubules lined by bland epithelial cells, reminiscent of renal tubules.
MUCINOUS TUBULAR AND SPINDLE CELL CARCINOMA
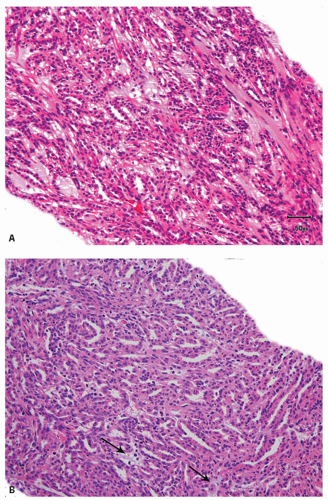
Unlike sarcomatoid RCCs, in spite of showing a spindle cell component, this distinctive tumor mostly shows an indolent clinical behavior (5,8,9,10); only a very occasional case with the typical histology has been described to show metastasis (11). Some mucinous tubular and spindle cell carcinoma (MTSCC) with high-grade sarcomatoid features or marked epithelial atypia have behaved aggressively, but such morphologic features are very rare (12,13,14,15). Because grossly most tumors are well-circumscribed, this circumscription may be noted even on core biopsies in the form of distinct demarcation from adjacent nonneoplastic renal parenchyma, often with an intervening distinct capsule. Microscopically, the tumor is composed of elongated, mostly straight and sometimes branching tubules with slit-like lumina or as solid compressed cords, and prominent low-grade spindle cell areas (Fig. 4.2A). The epithelialappearing cells are usually low cuboidal with small amount of amphophilic to eosinophilic cytoplasm and low-grade nuclei. The nuclei in the spindle cells are morphologically similar to those in the tubular areas. Uncommon features that may occasionally be seen include the presence of foamy macrophages, papillations, necrosis, and focal clear cells in tubules and rarely oncocytic tubules (16,17) (eFig. 4.1). Myxoid stroma is present in majority of the cases, although occasional tumors may be “mucin-poor” (Fig. 4.2B) (16).
Ancillary Studies
Immunohistochemical stains for CK7, racemase (AMACR), RCC antigen, and CD15 are positive in both the tubular and spindle cell areas. CD10
is mostly negative but may be focally positive in some cases (10,18). Ultrastructural evaluation, performed on a few cases, shows close resemblance to the loop of Henle (8). Comparative genomic hybridization (CGH) data available on a few cases shows frequent losses at chromosomes 1, 4q, 6, 8p, 11q, 13, 14, and 15, with gains at 11q, 16q, 17, and
20q (15). No VHL deletions or trisomy 7 and 17, and loss of Y have been found (19).
is mostly negative but may be focally positive in some cases (10,18). Ultrastructural evaluation, performed on a few cases, shows close resemblance to the loop of Henle (8). Comparative genomic hybridization (CGH) data available on a few cases shows frequent losses at chromosomes 1, 4q, 6, 8p, 11q, 13, 14, and 15, with gains at 11q, 16q, 17, and
20q (15). No VHL deletions or trisomy 7 and 17, and loss of Y have been found (19).
Differential Diagnosis
The main differential diagnostic consideration is type 1 papillary RCC with predominantly tubular architecture. Because of the morphologic and immunohistochemical overlap (18), it has been suggested that MTSCC may be a variant of papillary RCC (20). Rare cases of papillary RCC variant that contain low-grade spindle cell areas are even closer morphologic mimics (21). However, in addition to the usual lack of mucinous stroma in papillary RCC, we find the frequent absence of CD10 in MTSCC quite useful in this distinction. Another helpful feature is that the luminal surfaces of MTSCC tubules are very often straight, whereas these show irregular contours (shaggy lumina) in papillary RCCs (22). Argani and colleagues (21) have also highlighted the use of fluorescence in situ hybridization (FISH) for trisomies 7 and 17 in the distinction of MTSCC from papillary RCC with low-grade spindle cell areas, although it remains to be confirmed on larger studies.
CLEAR CELL PAPILLARY RENAL CELL CARCINOMA
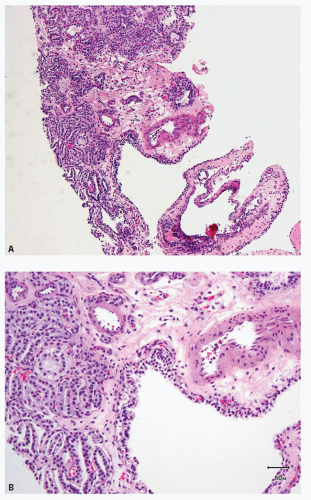
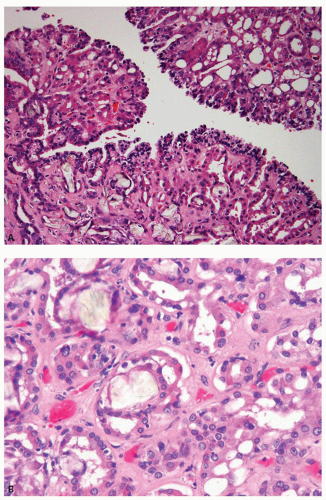
Clear cell papillary RCC initially described by Tickoo et al. (23) in the setting of end-stage kidneys, also occurs without evidence of impaired renal function (24,25,26,27,28,29). Although originally believed to be a morphologic variant of conventional clear cell carcinoma, published series reveal no evidence of 3p25.3 losses or VHL gene mutations, supporting it as a distinct entity (24,26,27,28,30). Additionally, polysomies of chromosomes 7 and 17, a common feature in papillary RCC, are not seen. These tumors are usually unifocal, unilateral, and small: the largest tumor described being 6 cm in maximum diameter. Grossly, they are well-circumscribed and often encapsulated, although the capsule may be of variable thickness. It is common for the lesion to be cystic or partially cystic; however, some are predominantly solid. Microscopically, the tumor cells have almost uniformly clear cytoplasm and low-grade nuclei (Fig. 4.3A,B). The cells are cuboidal and arranged in tight tubular/acinar structures, particularly in the solid areas. Very often the tubules show branching, resulting in the formation of apparent papillae (Fig. 4.3B). If the tumor cells have minimal cytoplasm, these areas can appear solid (collapsed acinar pattern) (Fig. 4.3C, eFig. 4.2). Sometimes papillary structures are seen tufting into cystic spaces that are also lined by clear cells. Characteristically, the tumor nuclei are arranged in a linear fashion, away from the basal aspect of the cell, either in the center or toward the apices of the cells (Fig. 4.3C,D). The nuclear arrangement and other cytologic features are easily identifiable in needle core biopsies. Stromal hyalinization and/or myoid metaplasia may
be evident within the lesion. Tumor necrosis and vascular invasion are not features of these tumors, although rare cases extending into the renal sinus have been described. Although the number of cases with extended clinical follow-up information are few in the literature, our experience suggests that these are indolent tumors.
be evident within the lesion. Tumor necrosis and vascular invasion are not features of these tumors, although rare cases extending into the renal sinus have been described. Although the number of cases with extended clinical follow-up information are few in the literature, our experience suggests that these are indolent tumors.
Ancillary Studies
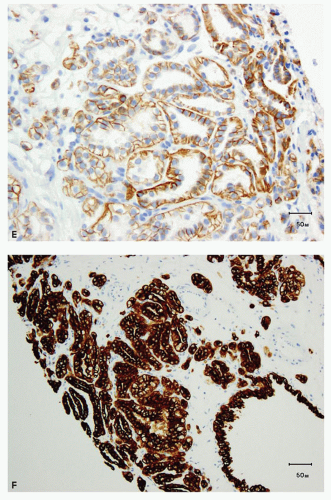
The immunohistochemical features of this tumor are quite characteristic (23,24,26,28,31). Tumor cells show diffuse membranous expression of CA-IX, but most often, there is absence of staining along the luminal borders of the tumor cells (cup-shaped distribution) (Fig. 4.3E, eFig. 4.3). CK7 shows diffuse immunoreactivity with invariable expression in almost 100% of the tumor cells (Fig. 4.3F). AMACR is entirely negative, whereas CD10 is negative in most cases (eFig. 4.4). It is common to see patchy to diffuse immunoreactivity for high-molecular-weight cytokeratin (34βE12) (eFig. 4.5).
Differential Diagnosis
The differential diagnosis include clear cell RCC (eFig. 4.6), papillary RCC, cystic nephroma/mixed epithelial stromal tumor (MEST), and benign multilocular cyst of the kidney. Attention to the cytologic features (in particular, the extensive linear arrangement of nuclei) as well as the unique immunohistochemical profile makes the distinction from clear cell RCC and papillary RCC relatively easy, even on needle core biopsies. Cystic nephromas/MEST are usually lined by a single layer of flattened or hobnailed epithelium, which only occasionally contains clear cytoplasm. The fibrous stroma may be more cellular, resembling ovarian stroma. The septa may contain small tubules lined by bland epithelial cells, reminiscent of renal tubules.
ACQUIRED CYSTIC DISEASE OF KIDNEY-ASSOCIATED RENAL CELL CARCINOMA
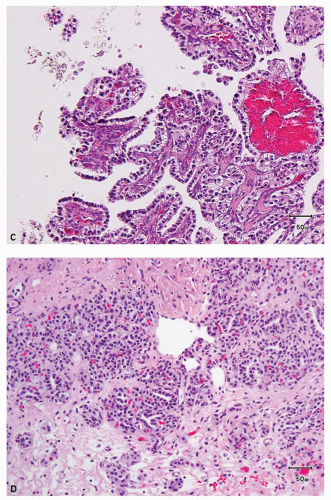
This recently described RCC is by definition associated with end-stage kidneys with acquired cystic disease (5,23). Most of the reported cases have occurred in patients on dialysis. Although the usual subtypes of renal epithelial tumors show an increased incidence in end-stage kidneys, acquired cystic disease of kidney (ACD)-associated RCC is the most common type of RCC arising in this setting (23). The tumor is characteristically composed of large eosinophilic cells with prominent nucleoli; inter- and intracellular spaces (holes), resulting in a vaguely cribriform architecture; and intratumoral oxalate crystals in a majority of the cases (Fig. 4.4A). Foci with clear or amphophilic cytoplasm may also be present. Architecture is variable and there may be papillary, acinar, tubular, cystic, and sheet-like areas in variable proportions (Fig. 4.4B). Some of these tumors behave aggressively, may metastasize, and cause death. Most of the aggressive tumors have sarcomatoid features (23,32); however, we have also observed rare nonsarcomatoid tumors that behaved in an aggressive manner and developed metastases.
Ancillary Studies
Immunohistochemical staining for AMACR and glutathione S-transferase alpha are diffusely and strongly positive, whereas CK7 is mostly
negative (23,33,34). Reported molecular studies revealed gains and losses of multiple chromosomes. Although gains of chromosomes 7 and/or 17 in some tumors have been reported, gains of chromosomes 1, 2, 3, 6, 7, 16, and Y were also frequently observed. Gains of chromosomes 3 and 16, in particular, have been among the more consistent findings (33,34,35,36,37).
negative (23,33,34). Reported molecular studies revealed gains and losses of multiple chromosomes. Although gains of chromosomes 7 and/or 17 in some tumors have been reported, gains of chromosomes 1, 2, 3, 6, 7, 16, and Y were also frequently observed. Gains of chromosomes 3 and 16, in particular, have been among the more consistent findings (33,34,35,36,37).
Differential Diagnosis
The variable architectural features may have led in the past to many of these being considered papillary, clear cell, or even unclassified RCC (22,23




Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree