the category of clear cell RCC in more recent years and are reclassified now as clear cell papillary RCC (26,27,28,29,30), translocation-associated RCC (31,32,33,34,35,36,37,38), multilocular cystic RCC/neoplasm of low malignant potential (25,39,40,41,42,43), and unclassified RCC, among others. Recognition of these specific clinicopathologic entities with differing biologic behaviors makes it imperative that on interpretation of needle core biopsies, such tumors are not misclassified as clear cell RCC (Table 3.2).
TABLE 3.1 Diagnostic Features of the Three Most Common Subtypes of Renal Cell Carcinoma on Needle Core Biopsies | ||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| ||||||||||||||
TABLE 3.2 Common Differential Diagnostic Considerations for the Three Most Common Subtypes of Renal Cell Carcinoma on Needle Core Biopsies | |||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| |||||||||||||||||||||||||||||||||
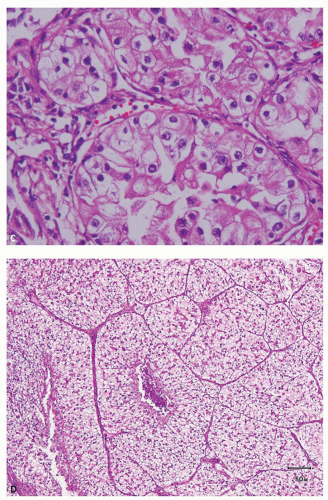
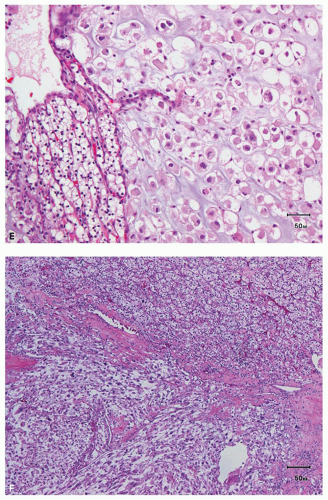
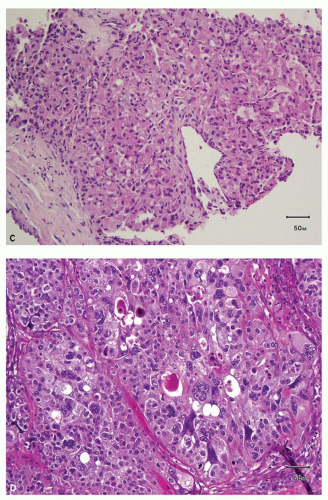
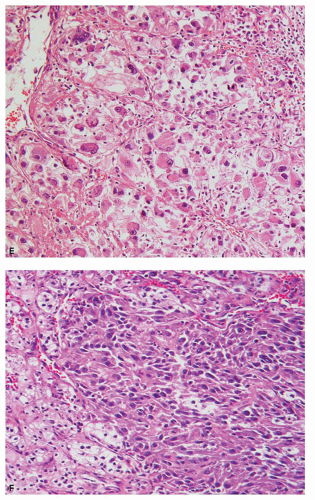
an acinar growth, whereas higher grade areas are more likely to exhibit solid, pseudopapillary, or sarcomatoid features (Fig. 3.2E,F). Tumors exhibiting a pseudopapillary growth pattern, which must not be confused with the true papillary growth pattern typically seen in papillary RCCs, are characterized by pseudopapillae lacking true fibrovascular cores. In our experience, pseudopapillations are particularly more common in core biopsies, especially at their edges, as these are the areas most prone to
lose the direct support and compression provided by the walls of biopsy needle on delivery from the needle. Such pseudopapillations occur more often in high-grade tumors, as they are less likely to have closely arranged fibrovascular septations and, therefore, abundant internal architectural support (Fig. 3.3). Occasionally, the biopsy may yield what at initial evaluation appears as fibrous tissue only. This often represents the biopsy needle
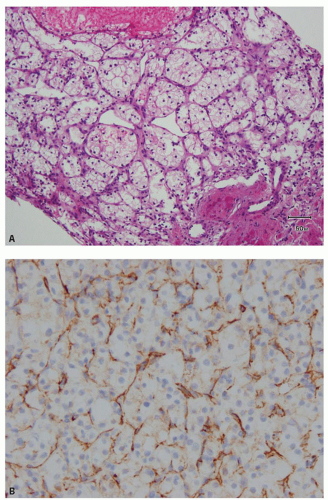
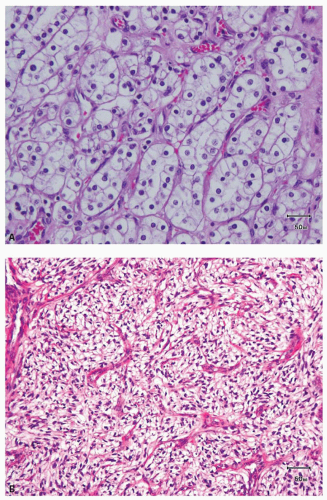
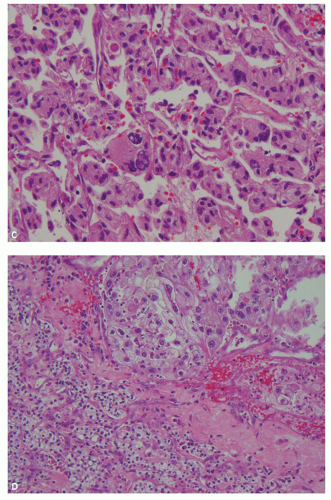
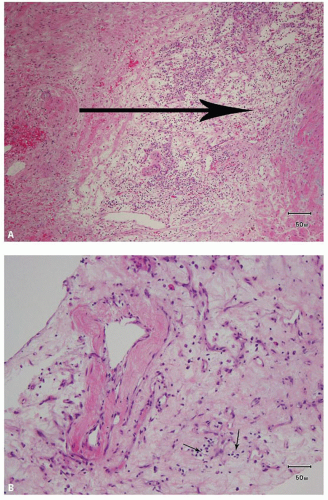
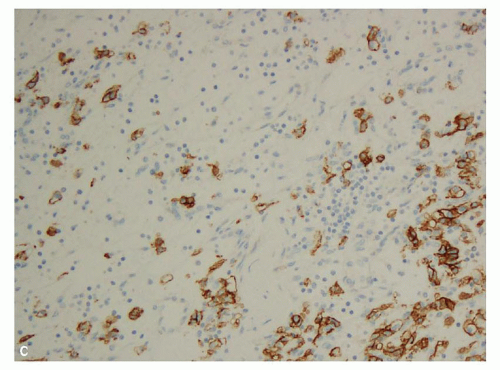
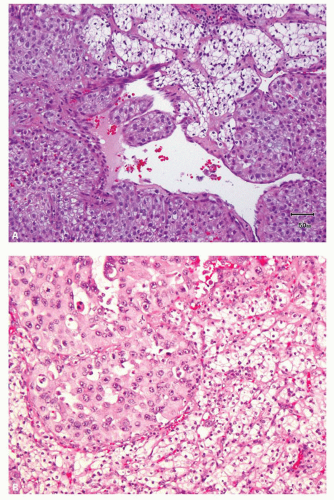
hitting a scar within the tumor. However, single or small clusters of cells— resembling small lymphocytes with scant clear cytoplasm—may often be noted dispersed within the fibrosis (Fig. 3.4A,B). These very frequently represent rare tumor cells that are still viable within scar tissue and must be investigated further, particularly with the help of immunohistochemical stains. The results on immunohistochemical staining in such material are frequently surprising as well as gratifying (Fig. 3.4C). Many of the tubules and acini show intraluminal hemorrhage—a characteristic but not specific finding in clear cell RCC (Fig. 3.5). This feature is common even at metastatic sites and should lead to the workup for a possible renal primary. Areas of necrosis, sometimes extensive (Fig. 3.6A), and hemorrhage are some other not uncommon histologic findings seen in core biopsies. Some tumors contain an inflammatory infiltrate, often predominantly lymphocytic in nature (Fig. 3.6B). Calcifications and osseous metaplasia, as well as intracytoplasmic hyaline globules or Paneth-like coarse granules (eFigs. 3.3 and 3.4) may also be seen in occasional biopsies.
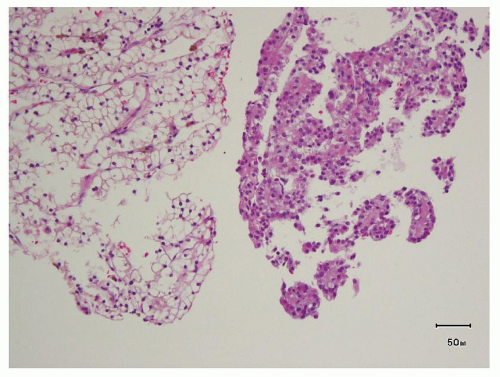 FIGURE 3.3 Pseudopapillations in an area of higher nuclear grade in a needle core biopsy from a clear cell RCC. |
4 lesions may altogether lack clear cytoplasm (Fig. 3.7C). Grade 4 lesions contain bizarre, pleomorphic and/or multilobulated, and multiple nuclei (Fig. 3.7D); cells with rhabdoid features (Fig. 3.7E); or spindle cells (sarcomatoid areas) (Fig. 3.7F).
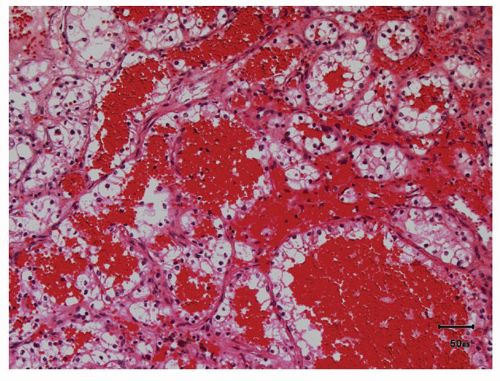 FIGURE 3.5 Pools of blood filling the acinar lumina, a characteristic, though not specific, feature of clear cell RCC. |
grade in biopsy often does not correlate with the final grade on resection specimen (7,44,45). Therefore, if the nuclear grade is provided, it should be qualified by a statement similar to “… in this limited material.” As is intuitive however, high nuclear grade in the biopsy tends to correlate with high grade on resection specimen as well (7,45).
well. Only rarely does clear cell RCC show focal presence of true papillary structures, but the usual typical acinar architecture is maintained in most of the tumor. CA-IX, CK7, and AMACR immunohistochemical stains will help in the distinction of clear cell RCC from papillary RCC in most cases. Unlike clear cell RCC, papillary RCC at the most shows
only patchy positivity for CA-IX, often limited to the tips of papillae or around areas of necrosis, whereas CK7 and AMACR are usually diffusely positive (20,47).
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree