Renal Biopsy and Normal Histology
Debra L. Zynger
Renal biopsies can be performed percutaneously, in which a needle core biopsy and/or fine needle aspiration is obtained, or endoscopically, in which the renal pelvis is sampled. Percutaneous biopsies are used primarily to examine renal cortical masses, whereas endoscopic biopsies are used for sampling renal pelvic lesions. This chapter addresses percutaneous renal biopsies with renal pelvic procedures discussed in Chapter 8.
INDICATIONS
A tissue diagnosis prior to surgical resection is the standard of care for most types of cancer, although this is not the case for tumors of the kidney. Use of a percutaneous renal biopsy in the workup of a renal mass has several established indications (Table 1.1). Patients with a renal lesion and a suspected extrarenal primary malignancy, such as lymphoma or lung carcinoma, may undergo a renal biopsy, as approximately half of these patients will have renal cell carcinoma (RCC) (1). In patients with suspected RCC that is surgically unresectable, renal tissue may be sampled in order to obtain a diagnosis, and the tumor subtype may be used to guide chemo- or targeted therapy (1,2). Renal insufficiency, a solitary kidney, and cardiac comorbidities render some patients to be poor surgical candidates. In these patients, it is prudent to determine the diagnosis before proceeding to surgery to be certain that the mass is malignant (1). Radiologic findings suggestive of infection or an inflammatory process such as xanthogranulomatous pyelonephritis may prompt a renal biopsy to ascertain the cause of the mass.
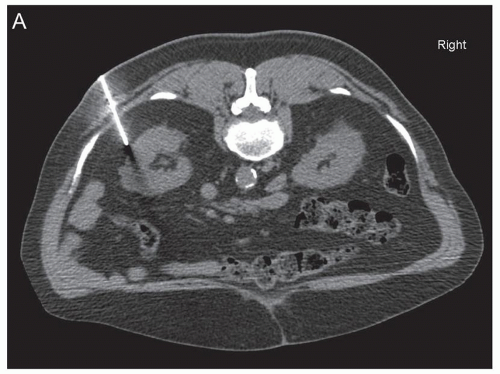
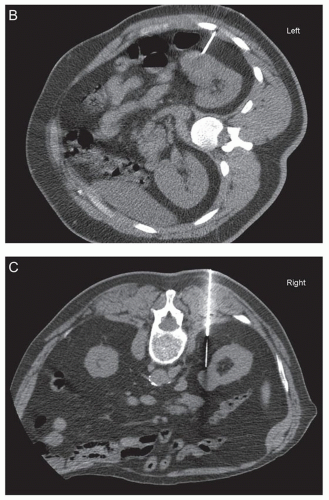
In addition to these established clinical scenarios, there are emerging indications in which a renal biopsy may be pursued. The increased use of abdominal imaging has led to more frequent detection of small (<3 to 4 cm) renal masses. Approximately 10% to 15% of solid renal masses are benign, with benign tumors proving to be oncocytoma in 70% of cases and angiomyolipoma in 20% (Fig. 1.1). In this subset with benign lesions, the morbidity and cost of surgery could be avoided via a diagnosis given
from a renal biopsy (3). Cystic masses may also be indeterminate upon imaging and thus a biopsy could assist in the diagnosis (4).
from a renal biopsy (3). Cystic masses may also be indeterminate upon imaging and thus a biopsy could assist in the diagnosis (4).
TABLE 1.1 Indications to Perform a Percutaneous Renal Biopsy | ||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| ||||||||||||||||||
Another emerging indication is due to in vivo ablative techniques being increasingly used. It is desirable to have a tissue sample prior to ablation, and this treatment may not be appropriate for patients with
high-grade RCC. A tissue diagnosis obtained from a renal core biopsy could result in the avoidance of ablation in those found to have benign tumors, provides a pathologic diagnosis for patients that do proceed to ablation, and directs patients with high-grade tumors to surgery (5,6,7). Additionally, a substantial proportion of suspected RCC (37%) referred for ablation has been proven to be benign using a percutaneous biopsy (5). Without a renal biopsy, these patients would have continued to have the
incorrect diagnosis of RCC and would contribute to an overestimated long-term cancer control efficacy of ablation (5). Following ablation, a renal biopsy may be performed to confirm success of the procedure and/or assess for recurrence subsequent to suspicious imaging (8).
high-grade RCC. A tissue diagnosis obtained from a renal core biopsy could result in the avoidance of ablation in those found to have benign tumors, provides a pathologic diagnosis for patients that do proceed to ablation, and directs patients with high-grade tumors to surgery (5,6,7). Additionally, a substantial proportion of suspected RCC (37%) referred for ablation has been proven to be benign using a percutaneous biopsy (5). Without a renal biopsy, these patients would have continued to have the
incorrect diagnosis of RCC and would contribute to an overestimated long-term cancer control efficacy of ablation (5). Following ablation, a renal biopsy may be performed to confirm success of the procedure and/or assess for recurrence subsequent to suspicious imaging (8).
Active surveillance is now a consideration, even for certain patients with RCC. This approach is reasonable as detection of small renal masses has increased the most in patients 70 to 89 years of age (Fig. 1.1) (9). The elderly population is more likely to have comorbidities limiting the feasibility of performing surgery safely, and also this cohort has a decreased life expectancy due to other non-renal tumor causes, offsetting the benefits of surgery. Supporting this notion, no survival benefit was identified for surgical resection of T1 RCC in patients older than 75 years of age (10). Active surveillance for a small mass diagnosed as RCC is an area which is currently evolving and involves combining the patient characteristics, tumor size, histopathologic diagnosis, and tumor growth rate as detected by serial imaging. Recently, a strategy in which renal tumors smaller than 4 cm are stratified into benign, favorable, intermediate, and unfavorable has been proposed (11). Using this strategy, chromophobe RCC, grade 1 papillary type 1 RCC, and grade 1 clear cell RCC are considered as the favorable group, and these undergo active surveillance. The intermediate risk group of grade 2 clear cell RCC, grade 2 papillary type 1 RCC, and oncocytic tumors not otherwise specified, undergo active surveillance if smaller than 2 cm and surgery if 2 to 4 cm. The unfavorable tumors, grades 3 to 4 clear cell RCC, papillary type 2 RCC, and RCC not otherwise specified, are managed surgically. Further research to define the optimal treatment algorithm, including active surveillance, is necessary.
PREBIOPSY STUDIES AND BIOPSY TECHNIQUE
Computed tomography (CT), ultrasound, and magnetic resonance imaging (MRI) are used to characterize renal masses, but only 17% of benign tumors are correctly diagnosed using imaging (12). Evidence for a benign lesion include the presence of intratumoral adipose indicative of angiomyolipoma or a nonenhancing lesion with smooth walls suggestive of a simple cyst. Most angiomyolipomas can be confidently diagnosed using imaging; however, fat-poor tumors may be misdiagnosed (2). A mass with ill-defined borders and/or perinephric stranding raises the possibility of pyelonephritis. Oncocytoma has characteristic CT imaging features including a homogeneous, hypervascular appearance with or without a central scar. However, many oncocytomas lack these classic features using CT or MRI, and a definitive diagnosis necessitates histologic sampling (2,13,14).
Prebiopsy workup includes clinical and laboratory assessment for bleeding risk (15). Most commonly, renal biopsies are obtained with the patient prone or semiprone or a lateral decubitus approach. CT or ultrasound
guidance or a combination of the two modalities is used (Fig. 1.1). Benefits of CT guidance include better visualization of the tumor and needle and thus easier avoidance of necrotic areas, but it is more costly, takes more time, and exposes the patient to radiation (6,16). Ultrasound guidance provides more flexibility with approach, can be used in multiple settings, has real-time imaging, and is lower in cost (1,6,16). However, bowel and the pleural space may be difficult to visualize. Both CT and ultrasound guidance have similar diagnostic yield, and the imaging selected is largely dependent on user preference and experience (17,18,19). Renal biopsy pathology specimens that may be collected include tissue cores, fine needle aspiration, or both. A variety of needle types and sizes (14 to 21 gauge) with coaxial sheaths are now commonly used (6,7). Cores that are smaller than 1 cm or are torn are stated to be unsatisfactory in the interventional radiology literature (6). At least two adequate biopsy cores are recommended (6). Central biopsies are reported to have worse yield, perhaps due to necrosis, and because of this, peripheral biopsies in larger tumors may provide better results (20). Larger core needles (14 to 21 gauge) are described to have better diagnostic yield than smaller (20 gauge) needles (21).
guidance or a combination of the two modalities is used (Fig. 1.1). Benefits of CT guidance include better visualization of the tumor and needle and thus easier avoidance of necrotic areas, but it is more costly, takes more time, and exposes the patient to radiation (6,16). Ultrasound guidance provides more flexibility with approach, can be used in multiple settings, has real-time imaging, and is lower in cost (1,6,16). However, bowel and the pleural space may be difficult to visualize. Both CT and ultrasound guidance have similar diagnostic yield, and the imaging selected is largely dependent on user preference and experience (17,18,19). Renal biopsy pathology specimens that may be collected include tissue cores, fine needle aspiration, or both. A variety of needle types and sizes (14 to 21 gauge) with coaxial sheaths are now commonly used (6,7). Cores that are smaller than 1 cm or are torn are stated to be unsatisfactory in the interventional radiology literature (6). At least two adequate biopsy cores are recommended (6). Central biopsies are reported to have worse yield, perhaps due to necrosis, and because of this, peripheral biopsies in larger tumors may provide better results (20). Larger core needles (14 to 21 gauge) are described to have better diagnostic yield than smaller (20 gauge) needles (21).
COMPLICATIONS
Percutaneous renal biopsy is a safe procedure. The most frequent complication is bleeding, but this is usually subclinical and self-limited. Approximately 90% of cases will demonstrate renal bleeding on CT imaging (22). Rare serious complications can occur. Bleeding requiring transfusion or hospitalization occurs in less than 1.5% of cases (2). Bleeding has yet to be associated with needle gauge size or number of passes taken, but an increased risk has been associated with poor renal function (23). Pneumothorax is a rare complication that can be avoided with a subcostal approach (2,24). Seeding of the needle track is exceptionally rare. It is seen in less than 0.01% of cases, with less than 10 cases documented in the literature (24




Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree