Study
Patients (n)
IORT dose (Gy)
5-y local recurrence (%)
5-y overall survival (%)
R0
R1
R2
R0
R1
R2
R0
R1
R2
R0
R1
R2
Haddock et al. (2009) [56]
227 (37)
224 (37)
156 (26)
12.5
15
20
28
32
32
46
27
16
Pacelli et al. (2009) [15]
–
–
–
10–15
10–15
10–15
–
–
–
–
–
–
Dreseen et al. (2008) [57]a
84 (57.2)
34 (23/1)
29 (19.7)
10
12.5
15–17.5
25
29.2
28.5
58.7
26.5
24.1
Heriot et al. (2007) [58]a
98 (61.3)
40 (25)
14 (8.8)
10
10
10
–
–
–
–
–
–
Hahnloser et al. (2002) [8]
138 (45)
27 (3.3)
139 (45.7)
–
–
–
–
–
–
27
–
–
Wiig et al. (2002) [59]
18
29
12
15
15
17.5–20
30
50
–
60
20
0
Shoup et al. (2002) [48]
64 (64)
30 (30)
6 (6)
12.5–15
15–17.5
15–17.5
–
–
–
31.2b
9b
14b
Haddock et al. [56] recently reported on a retrospective analysis of 607 patients with recurrent colorectal cancer who received IORT. IORT was preceded or followed by external radiation in 583 patients (96 %), 70 % of whom had tumors located within the pelvis. The median IORT dose was 15 Gy (range, 7.5–30 Gy). Survival estimates at 5 years were 46, 27, and 16 % for R0, R1, and R2 resections, respectively. On multivariate analysis, R0 resection was the only independent factor associated with improved survival. Although no randomized trials evaluating IORT have been performed to date, data from large single institution studies suggest that IORT may influence local control and survival. As one would expect, multiple studies suggest that the extent of surgical resection, and therefore the volume of residual disease, is an important factor in improving local control with IORT. The experience with intraoperative brachytherapy at the Memorial Sloan-Kettering Cancer Center was reported by Alektiar et al. [62] in a study of 74 patients treated from 1992 to 1998. Median follow-up was 22 months. Fifty of these patients had negative margin (R0) resection. Five-year local control was 39 %; 5-year disease-free and overall survival was 23 %. Negative margins predicted local control: a 5-year rate of 43 % in patients with R0 resection vs. 26 % in those with R1 resection. Patients with negative margins had 5-year survival of 36 %, compared to only 11 % in patients with positive margins. More recently, Dresen et al. [57] reported on 57 patients receiving re-irradiation of 30.6 Gy with IORT, in addition to preoperative re-irradiation with external beam radiotherapy. The IORT dose was dependent upon completeness of resection. Five-year overall survival was 48 % in patients with an R0 resection. On univariate analysis, R0 resection was more likely in patients receiving re-irradiation, compared to patients who had previously received radiotherapy and were treated with surgery alone. In addition, patients who were re-irradiated with IORT had improved overall survival and decreased local and distant recurrence. Radical resection and stage of the primary tumor were the only factors predicting overall survival on multivariate analysis [56].
The morbidities associated with IORT are generally acceptable, but may be difficult to distinguish from disease-related toxicity. Common side effects include wound infection, ureteral obstruction, gastrointestinal complications such as obstruction or fistula, and peripheral neuropathy. In the series reported by Alektiar et al., morbidities included wound complications (24 %), bladder complications (20 %), ureteral stricturing (23 %), and peripheral neuropathy (16 %) [62]. In the study of over 600 patients by Haddock et al. [56], 32 % of patients developed neuropathy, the most common radiation-induced toxicity. Seven patients developed ureteral narrowing or obstruction.
We currently use IORT in cases in which there are anticipated close margins. Care must be taken to shield radiation-sensitive structures; input from the surgeon is critical.
Surgical Technique
Key Concept: Distinguishing tumor invasion from adherence is difficult, and wide resection provides the best chance for a margin negative resection. While it is important to preserve as much healthy anatomy as possible, these procedures typically require extensive resection and subsequent surgical reconstruction.
In order to achieve complete resection of tumor with negative margins, all organs involved by tumor must also be resected. Therefore, these extensive procedures often require the coordinated involvement of surgical specialists in urology, gynecology, orthopedics, neurology, radiation oncology, vascular surgery, and plastic surgery. In the absence of the rectum after APR, recurrent cancers are more likely to invade adjacent organs such as the sacrum and sacral nerves posteriorly, the vagina and uterus, or seminal vesicles and prostate, and the bladder anteriorly, and the ureters, autonomic nerve plexus, internal ileac lymph nodes, and vessels laterally.
Tumor that adheres to regional anatomic structures is generally assumed to invade them; all or part of these organs must be removed en bloc with the tumor. Focal invasion of adjacent organs, or metastatic lymph nodes in the pelvic sidewall, requires extended resection. The type of procedure—total pelvic exenteration, posterior exenteration, anterior exenteration, APR with sacrectomy, and sacropelvic exenteration—depends on the extent of tumor spread as well as distance of tumor from the anal sphincter musculature.
Preoperative Regimen
Key Concept: Developing a routine is important to achieving intraoperative success and minimizing morbidity.
Preoperative evaluation, including physical examination and imaging, will determine the need for additional studies such as pelvic ultrasound, cystoscopy, or dedicated sacral bone evaluation. Cystoscopy may be performed before resection or intraoperatively. Placement of ureteral stents can be done preoperatively to help identify and protect the ureters. Patients undergo bowel prep the day before surgery. Antibiotics are delivered in the operating room along with anesthesia. The patient is placed in the lithotomy position, giving the surgeon anterior access to the pelvis and perineum. Surgery will be performed in one or two stages, depending on the type of resection.
Rectal Washout
Key Concept: Rectal washout has theoretical advantages to reduce tumor shedding, with minimal downside.
The practice of rectal washout remains controversial. Some have theorized that viable exfoliated tumor cells implant at distant sites of bowel mucosa, potentially resulting in some anastomotic and/or various locoregional recurrences. A few studies suggest that free malignant cells collect on circular stapling devices during anterior resection [63, 64], implanting during construction of the anastomosis. A number of small studies suggest that rectal irrigation may eliminate the free cells collected on circular staplers, reducing implantation and potential spillage into the pelvis [65, 66]. The type of rectal irrigation—saline vs. cytocidal—also remains a point of contention. Although cytocidal rectal washouts comprising solutions such as cetrimide or povidone-iodine are used more commonly, there is no data confirming that these are more effective than simple saline wash. A study by Church et al. concluded that rectal irrigation probably eliminates exfoliated malignant cells by mechanical cleansing, rather than through any cytocidal effect [67]. Similarly, Jenner and colleagues showed that saline wash effectively removes exfoliated malignant cells from the distal rectum mechanically [68]. Even so, no study to date has demonstrated the clinical relevance of rectal washout in reducing the incidence of local recurrence. In 2005, the American Society of Colon and Rectal Surgeons published practice parameters for the management of rectal cancer, stating that there was insufficient evidence to recommend intraoperative rectal washout [69]. However, given the minimal time involved and lack of detriment to the impending procedure, it is our practice to irrigate the rectum with 500 cc of 5 % povidone-iodine solution prior to incision.
Resection
Key Concept: You must maintain flexibility during the operation. This includes making an early decision as to whether you have the ability to perform an adequate resection that will benefit the patient.
Intraoperatively, you should first examine the abdomen for disseminated peritoneal disease, which would prevent a curative resection. This can be done via diagnostic laparoscopy, when possible, thus avoiding the morbidity associated with a major midline laparotomy. A laparotomy is often necessary, however, especially in the setting of adhesions.
The retroperitoneal lymph nodes should be examined for metastasis, which—especially if the nodes cannot be completely removed—may indicate incurable disease. The ureters are identified and preserved, and will not be transected until resectability is confirmed. Following abdominal inspection, the recurrent tumor is assessed. Dissection ideally begins in an extraperitoneal plane free of adhesions and scar tissue (Fig. 15.1). The inferior mesenteric artery is ligated and transected, followed by transection of the descending colon. The surgeon dissects posteriorly down to the levator ani, taking care to avoid the pelvic nerves whenever possible. The bladder is now mobilized from the retropubic space (Fig. 15.2). The bladder pillars attached to the lateral pubic rami are transected. In a female patient, the cardinal supporting ligaments are ligated and transected at the pelvic sidewall. In a male patient, dissection continues anteriorly and includes the prostate.
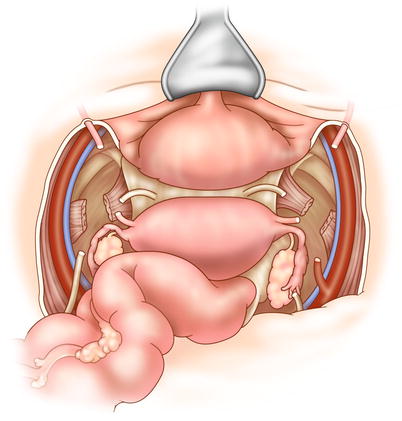
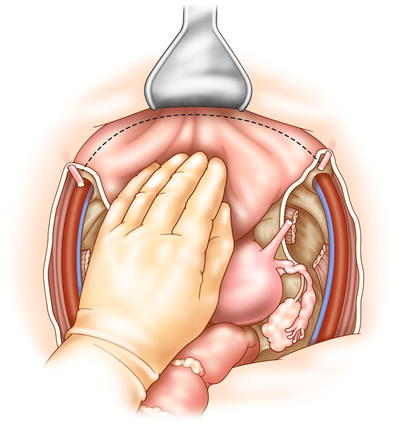

Fig. 15.1
In TPE, lateral dissection begins on the common and external iliac vessels, which are lateral to the parietal layer of the endopelvic fascia. The internal iliac artery and vein are clamped, cut, and tied distal at their origin. The ureter is cut in the pelvis, with care taken to preserve ureteral length for reconstruction

Fig. 15.2
The surgeon may perform dissection of the bladder before or after posterior dissection of the pelvic organs. The bladder is dissected from the symphysis and pubic rami, with dissection in the space of Retzius. The bladder is freed by dividing the lateral peritoneal attachments
A decision must now be made. Will you proceed with a low anterior resection, or an APR? In recurrent rectal cancer, an APR is usually necessary. If it is determined that an APR is required, dissection continues to the levator ani muscles, and then perineal dissection begins. The anal canal and lower rectum are dissected and removed through the ischiorectal fossa and urogenital diaphragm (Fig. 15.3). Wide lateral dissection of the pelvic floor (cylindrical dissection) is necessary to clear tumor. If tumor is extensively invasive in a female patient, the vagina, vulva, and urethra may have to be removed. The entire specimen can then be extracted through an abdominal or perineal incision.
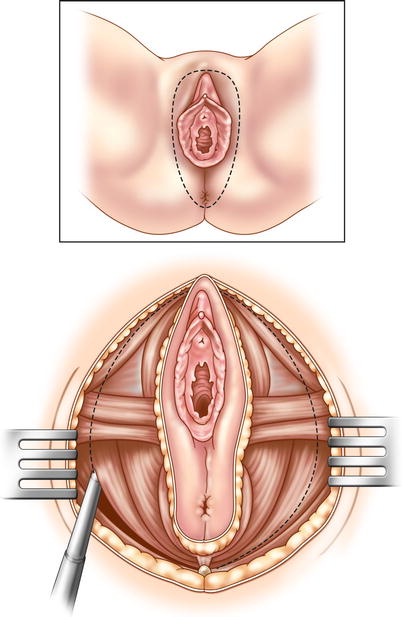

Fig. 15.3
Perineal dissection is necessary in TPE that includes the intra-levator organs (anal canal, labia majora, urethra). An elliptical incision is made from the tip of the coccyx to the pubic symphysis. The incision ends at the bulb of the penis (in a male patient), with the urethra previously divided in the pelvis. The pelvic floor attachments are divided widely, freeing the vagina (in a female patient), the urethra, and the rectum
Types of Procedures
Key Concept: The tumor location and extent of invasion will determine the type of procedure you perform.
Total exenteration is usually done in the setting of large, bulky lesions that invade the bladder or prostate. This procedure involves removal of the rectum, bladder, prostate, and seminal vesicles in male patients, and removal of the rectum, bladder, vagina, uterus, cervix, and parametrium in female patients.
Anterior exenteration is done when cancer invades the posterior bladder wall, anterior uterine wall, and organs in the anterior plane of the pelvis.
Posterior exenteration is done in a female patient if tumor invades the uterus. This procedure can be accomplished only if the bladder is not involved by tumor. Uterus, cervix, adnexa, and vagina (if required) are removed with the rectum. The operation is similar to total exenteration; however, instead of dissecting anterior to the bladder in the retropubic space, the peritoneum is incised over the bladder, and the bladder is dissected sharply off the anterior surface of the cervix and vagina and (depending on the level of tumor) down to or beyond the levator ani muscles. The ureters are dissected free from the anterior parametria distally, over the ureteral tunnel running along the uterine artery.
APR or LAR with partial cystectomy or vaginectomy may be considered if tumor does not extend into the bladder [involving the trigone] or the vagina far enough to require total removal of these organs. A partial cystectomy and reimplantation of the ureters can be done with a psoas hitch reconstruction. If only part of the vagina is involved by tumor, local resection of the invaded portion may suffice. If the resulting vaginal defect is too large for primary closure, reconstruction can be achieved using a myocutaneous rectus abdominis flap.
Sacral Resections
Key Concept: Sacral resections are generally done if tumor broadly adheres to or invades the sacrum or coccyx.
APR with sacrectomy begins in the same manner as a total pelvic exenteration: dissection in the ventral plane anteriorly, preserving the bladder, female reproductive organs, or prostate, if possible. Dissection takes place in a dorsal and dorsolateral fashion, following the presacral plane down to the level of the sacral transection. If transection of the sacrum at the S2/S3 level (or lower) clears disease, the cancer is resectable. Resection above S2 involves significant morbidity; the need for tumor clearance at that level often indicates unresectable disease. The level of sacral transection is marked on the anterior cortex of the sacrum using osteotome or K-wire. Gauze may be packed into the presacral space to reduce bleeding. The patient is turned and placed in the prone position. A dorsal longitudinal incision is made, starting at the level of L5 down to and around the anal canal. The gluteus maximus and gluteus minimus muscles are dissected off the sacrum, and the flaps are raised bilaterally. Transection of the sacrotuberous and sacrospinous ligaments is done at the sacrum, facilitating access to the pelvic floor muscles and infra-piriformis opening. Medial to the infra-piriformis, you should insert a finger into the presacral space to identify the level of resection (Fig. 15.4). The sacrum is now resected, with care taken to protect the nerve roots within the proximal (preserved) sacrum. The distal sacrum, lateral pelvic walls, and rectum are removed en bloc.
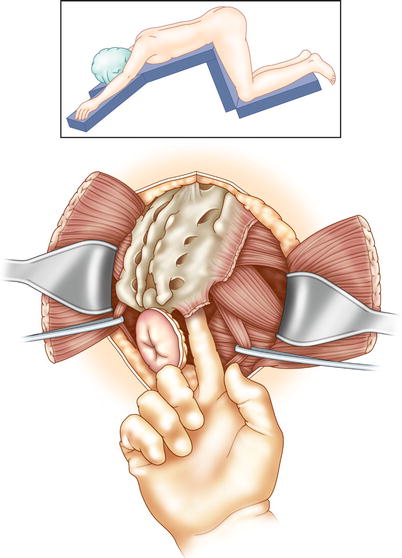

Fig. 15.4
After anterior dissection, the patient is placed in the prone position for sacral resection. A posterior sacral incision is made with excision of the anus. Flaps are raised to the lateral extent of the sacrum. The gluteus maximus and gluteus medius muscles are dissected from their sacral origins. The sciatic nerve is located by retracting the gluteus maximus and underlying piriformis muscle superiorly, at the lateral aspect of the mid-sacrum. The nerve is superficial to the obturator internus muscle, coursing inferolaterally between the ischial tuberosity and greater trochanter. The sacrotuberous and sacrospinous ligaments are incised at their attachments to the ischial tuberosity and ischial spine. The surgeon inserts a finger anteriorly from the medial aspect of the sciatic nerve, facilitating dissection beneath the piriformis muscle and through the underlying endopelvic fascia. This exposure directs the sacral ostectomy, ensuring sufficient tumor clearance
Sacropelvic exenteration is undertaken only in the setting of very bulky tumors involving the lower sacrum and invading the reproductive organs in a female patient, the prostate in a male patient, and the bladder. This is a two-stage procedure: posterior dissection for distal sacrectomy and anterior dissection for pelvic exenteration. In the second stage, the patient is turned and placed in the prone position [70]. After division of the sacrum in stage two, the rectum is removed in continuity with the sacrum and resected visceral organs.
Pelvic Floor Reconstruction
Key Concept: Following resection of bowel, bladder, vagina, and perineum, the resultant defect will typically require reconstruction entailing multidisciplinary help and meticulous preoperative planning.
The major goals of reconstruction are to optimize healing, prevent perineal sepsis, and, in some cases, restore function. Type of reconstruction depends on the nature and extent of the surgical resection. If the external sphincter muscles have been left intact, the colon can be anastomosed to the distal rectum or anal canal. Because anastomotic leak is probable after such extensive treatment, a defunctioning ileostomy is always recommended. In most circumstances, rectal anastomosis is not possible, and a permanent colostomy is created. You will then normally confront a large, irradiated pelvic “dead space” susceptible to abscess formation and wound-healing complications. This area should be filled with vascular tissue such as omentum or a rotated myocutaneous flap [71–73]. Prosthetic or biological meshes have also been used, but are not favored by the authors due to risk of infection. Reconstruction of large vaginal defects, or defects in the perineal skin, is best accomplished with myocutaneous flaps [71]. If a cystectomy is done, options for urinary diversion include an ileal conduit or an orthotopic bladder substitution. Colon or ileum may be used for continent diversion (i.e., Indiana pouch, Mainz pouch, Florida pouch, Miami pouch). An ileal conduit, colonic conduit, or ureterocolostomy can also be constructed for urinary diversion [70].
Postoperative Complications
Key Concept: Due to the nature of the operation required for optimal outcomes, morbidity rates are significant, and you should have a plan for early identification and management of morbidity.
Most of the recent literature reporting on radical resection for locally recurrent rectal cancer describes acceptable perioperative mortality but significant morbidity (Table 15.2). Potential morbidities include surgical site infection, sepsis (usually related to the non-collapsible empty pelvis), complications related to urinary diversion, and complications related to IORT, including peripheral neuropathy and ureteral stenosis (Table 15.3). Dresen et al. [57] reported an overall complication rate of 59 % in their series of 144 patients undergoing radical resection for local recurrence. Nineteen percent suffered urinary retention and required prolonged catheterization. Fifteen percent developed pelvic abscess, requiring intervention. In another series of 160 patients undergoing radical or extended radical resection for recurrence, Heriot et al. [58] reported a relatively low morbidity of 27 % and minimal mortality of 0.6 %. Although it was not statistically significant, complications were more common in patients undergoing extended resections (requiring removal of at least one of the adjacent organs); the majority of these were perineal wound complications or pelvic abscess. A study from the Mayo Clinic reported 0.3 % in-hospital mortality and a 32 % rate of major complications in 304 patients undergoing resection for recurrent rectal cancer [8].
Table 15.2
Postoperative complications (%)
3.1–13 | |
5.3–13 | |
4–26 | |
4.4–23 | |
1.2–4.3 | |
4.6–9.4 | |
Neuropathy [56] | 15 |
Table 15.3
Summary of long-term outcomes for multimodality therapy of recurrent rectal cancer
Study | Pts | Resected | Cases (%R0) | IORT +/− (n) | EBRT +/− (n) | Median survival (mos) | Morb. (%) | Mort. 30 d (%) | LR (%) | LR (R0) | DR (%) | OS (%) | 5-y OS R0 |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
Haddock et al. (2009) [56] | 607a | 427 (rectum) | 37 | + (586) | + (228) | 36 | 50 | 1 | 28 | 28 | 53 | 30 | 46 |
Pacelli et al. (2009) [15] | 58 | 44 | 62.5 | + (20) | + | − | 20.9 | 7 | 25.7 | 11.5 | − | 54.2 | 72.4 |
Hansen et al. (2009) [74] | 577 | 185 | 52.4 | − | + | 48 (R0) | − | 1.6 | − | 17.5 | 25.1 | 14.9 | 62 |
Dresen et al. (2008) [57] | 184 | 147 | 57.2 | + (136) | + (39) | 28 | 58.5 | 4.8 % | 45.9 | 31 | 20.4b | 28 | 48.4 |
Heriot et al. (2007) [58] | 160 | 153 | 61.2 | + (12) | − | 43 | 27 | 0.6 | − | − | − | 36.6 | 50 |
Asoglu et al. (2007) [7] | 72 | 50 | 48 | − | − | 19 | 24 | 0.0 | 12.5 | 33 | 33.3 | 33 | 36 |
Boyle et al. (2005) [45] | 64 | 57 | 36.8 | − | − | 33.6 | 43.9 | 1.6 | − | 49 | − | 40b | − |
Valentini et al. (2004) [52] | 59 | 30 | 35.6 | − | + (59) | 42 | 15.4 | 2.6 | 31 | 17.8 | 30.5 | 39.3 | 66.8 |
Hahnloser et al. (2002) [8] | 429 | 304 | 45 | + (131) | + (244) | 31 | 32 | 0.3 | − | − | − | 25 | 37 |
Wiig et al. (2002) [59] | 107 | 107 | 36 | + (59) | + | 40 | 44 | 1.6 | 50 | 30 | − | 30 | 60 |
Shoup et al. (2002) [48] | 634c | 111 | 64 | + (111) | − | 31.2 | − | 0 | 33 | − | 45 | 22d | 31.2d |
Complications and perioperative mortality increase with more radical procedures, such as sacropelvic resection. In 1994, Wanebo et al. reported 8.5 % perioperative mortality in 47 patients undergoing exenteration for recurrent rectal cancer [75]. The majority of complications were related to perineal and abdominal wound sepsis. It should be noted that a significant proportion of these procedures involved relatively high sacrectomies (S1 and S2). A 2006 study from the Memorial Sloan-Kettering Cancer Center reported on complications following sacropelvic resection in 29 patients with recurrent rectal cancer. Sacral resection was performed at the S2/S3 level in 55 % and at the S4/S5 level in 45 % of the study cohort. Previous surgery predicted the type of salvage operation required: total exenteration with sacrectomy was performed in 69 % of patients who had previously undergone APR; a less radical procedure was done for those who had undergone sphincter-saving surgery. In 59 % of patients, pedicle flaps were used to reconstruct the pelvis. The total complication rate was 59 %; 45 % were major complications, and most involved perineal wound breakdown and pelvic sepsis. There was one perioperative death [76].
Stoma
Key Concept: Permanent or temporary diversion is routine in these cases and is associated with its own set of complications, which you should be prepared to manage. Proper marking, preoperative involvement of an enterostomal therapist, and adequate technical construction can minimize stoma-related morbidity.
The reported incidence of ostomy complications has varied over the past three decades, ranging from 14 to 70 % [77–81]. Retrospective studies have identified several risk factors associated with increased overall stomal complications: poor perioperative siting, lack of stoma education by an enterostomal therapist [82, 83], height of the stoma (<10 mm) [84], creation of a stoma after emergency surgery [78], and patient comorbidities such as obesity [83, 85], Crohn’s disease [83], and advanced age. Diabetes and smoking are associated with poor wound healing and, on several univariate analyses, have been found to play a potential role in ostomy separation, retraction, and parastomal hernia [78, 86].
Stoma-related complications can be categorized as early or late. The most common complications occur in the immediate postoperative period (less than 30 days) and include peristomal skin breakdown, stomal retraction, stomal necrosis, mucocutaneous separation, poor location, surgical wound infection, and sepsis. Late complications (typically 6–12 weeks after surgery) include parastomal hernia, prolapse, retraction, stenosis, obstruction, and stomal bleeding.
Preoperative counseling has been shown to help patients adapt psychologically to the significant lifestyle changes associated with having a stoma. Patients experience physical, psychosocial, and emotional stress, often because of changes in sexual function, self-esteem, social acceptance, and economic burden [87]. An enterostomal therapist should educate patients about their upcoming surgery and prepare them for what to expect afterwards. Counseling is not limited to preoperative education but also includes stoma site selection, pre- and postoperative technical advice, emotional support for the patient and family, discharge planning, outpatient follow-up, and ongoing rehabilitation [88]. Appropriate preoperative counseling has been associated with decreased stoma-related complications [78, 82], better postoperative stoma proficiency, earlier discharge from hospital [89], and overall improvement in quality of life.
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree








