of 8.4% from the Australia and New Zealand (ANZ) Transplant Registry.
TABLE 13.1. Classification for recurrent and de novo diseases after renal transplantation | |||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
|
a crude grid of recurrence rather than actuarial rates, which are dependent on other causes of graft failure such as death acute and chronic rejection. In some studies, the rate of histologic recurrence was reported; while in others, patients with clinical presentation suggestive of recurrence was defined as recurrence. The rate of recurrence is not only dependent on the definition of recurrence but also on the follow-up after transplantation. Large databases such as USRDS and UNOS report only recipients who lose their graft due to recurrence, underscoring the true prevalence of recurrence. Prevalence of recurrent disease is also dependent on the ESRD population. For instance, prior to 1980s only nondiabetic patients were accepted for renal transplantation in most centers, thus the prevalence was higher with predominant GN recipients undergoing transplantation. However, in recent years nearly 50% of recipients have diabetes as the cause of ESRD, and smaller proportion of patients with GN are undergoing transplantation. Due to a very low incidence of most recurrence disease, many studies lack statistical power to directly assess the incidence and risk factors for recurrence. All these factors make it difficult to estimate the true incidence of recurrent diseases after renal transplantation, and in the end only very crude estimates can be provided.
TABLE 13.2. Risk of recurrence | ||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| ||||||||||||||||||||||||||
and this has been associated with poor short- and long-term survival (17, 18, 19). Native disease types such as those who develop rapid progression toward ESRD, collapsing variant, and children have the highest chance of developing recurrence. Recipients who have graft failure due to recurrence after their first transplant have an 80% chance of developing recurrence after their second transplant. Savin et al have postulated a serum factor correlating recurrence of FSGS (12).
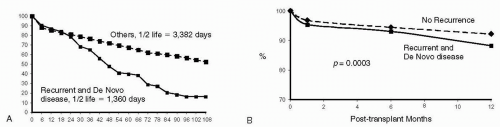 FIG. 13.1. A. Lower long-term graft survival in recipients with recurrent and de novo graft disease. B. Lower short-term graft survival in recipients with recurrent and de novo disease. |
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree







