Fig. 8.1
Workup and treatment algorithm for rectourethral fistulas
Retrograde urethrogram (RUG) may be may be performed while the patient is under anesthesia or in the office setting. This will aid in further delineating the location and size of the fistulous tract. If done with the patient awake in the office, concomitant voiding cystourethrogram (VCUG) should also be performed (Fig. 8.2). This will provide additional information with regard to bladder neck and posterior urethral pathology, such as urethrovesical anastomotic stenosis or prostatic urethral stricture.
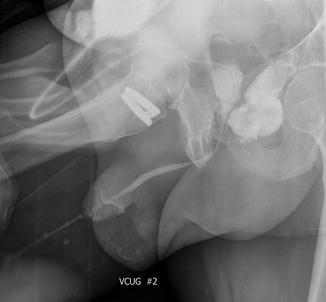

Fig. 8.2
Voiding cystourethrogram demonstrating a rectourethral fistula
Cross sectional abdominopelvic imaging using either computed tomography (CT) or magnetic resonance imaging (MRI) may be indicated in cases where the standard workup is insufficient and the anatomy of the fistula is not clear. This imaging is also helpful in men with prior failed repairs. Some have also suggested that MRI allows demonstration of an intervening cavity between the rectum and urethra, which may aid in surgical planning and patient counseling [21].
Urodynamic testing is occasionally helpful in the evaluation of a rectourethral fistula, though they are often difficult to perform depending on the size of the fistula tract and volume of urine leakage. If they are performed, an assessment of bladder capacity is helpful. Men with radiation induced RUF will frequently also have a reduced bladder capacity. As a consequence, some will be better served with a cystectomy and urinary diversion rather than attempts at fistula repair.
Lastly, men presenting with urorectal fistulas should be evaluated for sexual function and overall quality of life. Erectile function is known to be poor in men with radiation induced RUF [11]. Men considering fistula surgery who have adequate erectile function should be counseled that surgical treatment might result in worsening of their erection quality.
Conservative and Endoscopic Management
Conservative management of rectourethral fistulas generally refers to transurethral or suprapubic urinary drainage in conjunction with a “low residue” diet or temporary colostomy to reduce rectal fecal burden. This regimen is continued for 4–6 weeks and followed by repeat fistula assessment with an office RUG and VCUG. Extending the trial of conservative management beyond 6 weeks for those with a persistent RUF would be futile and those patients should be counseled on surgical options at that time.
Any attempts at conservative management of rectourethral fistulas should reserved for those men with small, surgically induced fistula without associated radiation or tissue ablation injury. This group of patients has the best chance for non-surgical resolution of their fistula. It is likely that rather than a true rectourethral fistula, this situation represents an iatrogenic urorectal communication that has not been present for sufficient time to form an epithelial tract. In this specific scenario, urinary diversion and dietary changes may allow healing to occur before an epithelialized communication becomes permanent.
Several authors have reported positive results using this conservative management technique in surgical RUF. Most recently, Thomas and colleagues reported on 12 patients with a surgically induced fistula who underwent attempts at conservative management with urinary drainage +/− diverting colostomy [2]. Of the 12 men, five (42 %) had resolution of their fistula without surgical intervention. Fecaluria was found to be a negative prognostic sign for fistula resolution with conservative treatment, suggesting that patients with fecaluria have larger and more complex fistulas. Others have also noted some success with conservative measures. Nyam and Pemberton demonstrated at 14 % success rate, and Al-Ali and associates a 46.5 % closure with a similar treatment paradigm [23, 24].
Contrary to an uncomplicated surgical fistula, however, men with a history of radiation or tissue ablation are significantly less likely experience spontaneous closure [5]. These fistulas are complicated by generally being larger in size with inflamed and poorly vascularized surrounding tissue. They also more often present in a delayed fashion when an epithelial tract has had ample time to establish. All of these factors contribute to lack of spontaneous closure.
In addition to urinary and fecal diversion, minimally invasive endoscopic treatments have been attempted for small fistulas. Dolay et al. published a successful case of successful RUF treatment with endoscopic injection of fibrin glue into the fistula tract and rectal mucosal clipping [25]. A similar case report demonstrated success injecting fibrin glue into a complex RUF secondary to rectal Crohn’s disease. The fistula resolved and had not recurred at 3 years of follow up [26]. We have also attempted a technique of injecting fibrin glue in men with small and uncomplicated RUF with some success, though small numbers. Fibrin glue theoretically works in these patients by occluding the fistula tract, promoting native fibrin deposition and stimulating fibroblast proliferation. It also stimulates epithelialization and neovascularity, all of which promote fistula resolution. This technique may be an option for those men with uncomplicated fistulas who fail conservative measures and either refuse or are not candidates for a definitive open repair. However, a standardized technique and more robust outcome data are necessary before this minimally invasive treatment option can be broadly recommended.
Open Surgical Management
The vast majority of rectourethral fistulas will require open surgical management. There are several basic surgical principles for optimization of outcomes with fistula repair. No matter which technique is chosen, complete excision of the fistula tract followed by a multilayer, tension free rectal and urethral closure is mandatory. In all but the simplest surgical fistulas, tissue interposition, usually accomplished with a local flap, will improve outcomes. Flaps are especially necessary for any redo procedures or large RUF associated with radiation and/or tissue ablation techniques [6]. Tissue interposition creates a space of separation between the prior fistulous communication and reduces the likelihood of fistula recurrence.
Timing of open repair is often dictated by surgeon preference and experience. It is generally our practice, and that of others, to wait 3 months after a diagnosis surgical fistula with urinary diversion +/− fecal diversion before proceeding with repair [6, 27, 28]. This allows the patient at least an attempt at spontaneous closure and gives time for tissue infection and inflammation to improve or resolve. In men with radiation induced fistulas or RUF following tissue ablation techniques we generally wait 4–6 months as the associated tissue inflammation and tissue necrosis is significantly increased in this group. Men presenting with sepsis or local infection must be adequately treated and fecal diversion is nearly always necessary in this group preoperatively. In those instances we will frequently delay repair slightly longer to allow sufficient tissue healing and resolution of infection.
Preoperative preparation depends on whether the patient has already undergone fecal diversion with a colostomy or ileostomy. If fecal diversion is planned as part of the fistula repair, a full polyethylene glycol mechanical bowel preparation ensures a stool free rectum during surgery. If a fecal diversion was performed prior to fistula repair this is unnecessary. IV antibiotics that cover both skin and gastrointestinal flora are administered within 1 h of incision. Patients with a prior fecal diversion can be fed immediately following surgery. Those undergoing diversion at the time of fistula repair or if electing to undergo repair without a covering fecal diversion should are kept NPO until return of bowel function.
Postoperative care depends on the fistula etiology. Urinary diversion is managed for all patients with a suprapubic tube (SPT) and Foley catheter following RUF repair. The Foley is kept in place for 3–4 weeks and a VCUG is performed at the time of Foley removal confirming the fistula resolution and absence of urethral stricture or bladder neck stenosis. If a fecal diversion is present, this is generally maintained for 3 months following fistula repair. Prior to reversing the diversion, repeat endoscopic and radiographic examination of the urethra is recommended to ensure complete resolution of the fistula tract.
Transanorectal (York Mason and Parks Procedure)
Historically colorectal surgeons rather than urologists performed the majority of rectourethral fistula repairs. As a consequence, surgical approaches utilized for other colorectal surgeries were more commonly used during fistula repair. Although innumerable techniques have been described, transanorectal procedure can broadly be divided into sphincter-splitting approaches (York Mason [29–31]) or more recently the sphincter-preserving transanal rectal advancement flaps (Parks procedure [32]).
Bevan was the first to describe transsphincteric rectal surgery in 1917 for rectal tumors [33]. Its application for the treatment of rectourethral fistulas, however, was not reported until 1969 by Kilpatrick and York Mason [29]. Transanorectal procedures begin by placing the patient in prone jackknife position. The buttocks are spread with adhesive tape. An incision is then made in the midline from the coccyx to the anal verge. The external sphincter is divided with care to place paired sutures at each level of the muscle. These sutures ensure proper sphincter alignment during reconstruction at the completion of the procedure. The rectum is then opened posteriorly along the incision, allowing exposure of the fistula tract. The fistula is sharply excised with a scalpel. Prospectively catheterizing the fistula tract can be helpful during this portion of the case, but is not mandatory. After the fistula and associated inflammatory tissue has been excised, the rectum and urethra mobilized to allow sufficient separation. A tension free, layered closure of the urethral and rectal defects is then performed with absorbable suture. Three layers of tissue are utilized. The urethra is closed first over a Foley catheter. A substantial layer of anterior rectal wall muscle is approximated second followed by the rectal mucosa, which comprises the third layer. The sphincter is reconstructed and the presacral and overlying tissues cover the defect.
The largest experience with the transanorectal modified York Mason approach to RUF repair was presented in 2012 by Hadley and colleagues from the University of Utah [34]. Fifty-one patients at their institution underwent this approach to fistula repair over their 40 year experience. Only seven patients had radiation-induced fistulas with the remainder surgical fistulas. To date they have only experience five fistula recurrences with a greater than 90 % success rate. One of the failures was salvaged with a repeat York Mason procedure. The remainder underwent permanent urinary or fecal diversion. A summary of outcomes from this and other select series using this technique can be found in Table 8.1.
Treatment approach | Tissue flap usage | ||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
Study | Year | Total RUF patients | H/o XRT or tissue ablation, n (%) | Temporary fecal diversion, n (%) | Resolution with conservative treatment, n (%) | Transanal, sphincter sparing, n (%) | Transanorectal, sphincter-splitting, n (%) | Transabdominal, n (%) | Transperineal, n (%) | Any, n (%) | Gracilis, n | Omentum, n | Other, n | Successful surgical RUF closure, n (%) | Mean follow up, months |
Hadley et al. | 2012 | 51 | 8 (16) | 20 (39) | – | – | 51 (100) | – | – | 2 (6) | 2 | – | – | 46/51 (90) | NR |
Garofalo et al. | 2003 | 23 | 3 (13) | 16 (70) | 4 (17) | 14 (61) | 3 (13) | 2 (9) | – | – | – | – | – | 13/19 (68) | 31 ± 33.4 |
Joshi et al. | 2011 | 5 | – | 3 (60) | – | 5 (100) | – | – | – | – | – | – | – | 4 (80) | 11 (4–24) |
Al-Ali et al. | 1997 | 30 | – | 30 (100) | 14 (47) | 1 (3) | 11 (37) | 3 (10) | 1 (3) | 2 (12.5) | – | 2 | – | 14/16 (87.5) | 66 (18–132) |
Rouanne et al. | 2011 | 10 | – | 10 (100) | – | – | 10 (100) | – | – | – | – | – | – | 10/10 (100) | 24 (18–28) |
Kasraeian et al. | 2009 | 12 | 2 (17) | 6 (50) | – | – | 12 (100) | – | – | – | – | – | – | 9/12 (75) | 22 (2–73) |
Vanni et al. | 2010 | 74 | 39 (53) | 73 (98.6) | – | – | – | – | 74 (100) | 74 (100) | 68 | – | 6 | 68/74 (92) | 20 |
Voelzke et al. | 2013 | 23 | 13 (57) | 21 (91) | – | – | – | – | 23 (100) | 7 (30) | 2 | – | 5 | 18/23 (78) | 13 (3–39) |
Ulrich et al. | 2009 | 26 | 14 (54) | 26 (100) | – | – | – | – | 26 (100) | 26 (100) | 26 | – | – | 26 (100) | 22 ± 14 |
Ghoniem et al. | 2008 | 25 | 17 (68) | 25 (100) | – | – | – | – | 25 (100) | 25 (100) | 25 | – | – | 25 (100) | 28 (3–132) |
Wexner et al. | 2008 | 36 | 20 (56) | 36 (100) | – | – | – | – | 36 (100) | 36 (100) | 36 | – | – | 28 (78) | NR |
Lane et al. | 2006 | 22 | 22 (100)
Stay updated, free articles. Join our Telegram channel
Full access? Get Clinical Tree
 Get Clinical Tree app for offline access
Get Clinical Tree app for offline access

| ||||||||||||





