Radiotherapy of Urologic Tumors: Introduction
The primary management of genitourologic malignant diseases has been tied to the use of radiation for more than 100 years. In 1895, Roentgen described x-rays; by 1899, a patient with skin cancer was cured with radiation; and within 10 years, radiation was used to treat prostate cancer (Pasteau and Degrais, 1914). Radiotherapy became a mainstay of treatment for bladder and testicular cancers and later prostate cancer as supervoltage sources became available. Although chemotherapy and aggressive surgery have supplanted some of the uses of radiotherapy, radiation continues to play a major role in the management of carcinomas of the penis, urethra, prostate, and bladder. In this chapter, we review general principles and the indications for using radiation as a component in the primary management of urologic malignant diseases. The role of radiation as an agent of palliation has been well documented elsewhere and is excluded from this chapter.
General Principles of Radiotherapy
The effects of radiation on tumor and surrounding normal tissues are thought to be mediated primarily through the induction of unrepaired double-strand breaks in DNA. Excited electron species generated in the presence of oxygen form peroxide radicals, which fix chemical lesions and result in the generation of either repairable or nonrepairable DNA double-strand breaks. High linear energy transfer radiation (including neutrons and heavy-charged particles) is associated with less repairable DNA damage. Classically, the expression of radiation damage is not seen until the target cells enter mitosis. Differentiated normal tissues with low mitotic activity, such as the heart and spinal cord, tend to express the effects of radiation much later than cells from more kinetically active tissues, such as the epithelial cells lining the rectum, bladder, or urethra. However, differentiated normal tissues with low mitotic activity are more sensitive to the use of high dose per fraction or high linear energy transfer radiotherapy. In organs in which the functional stromal cells are postmitotic, such as muscle cells and neurons, the damage is expressed by slowly dividing support cells such as endothelial cells.
In addition to the classic mechanism described earlier, radiation has been shown to induce programed cell death (apoptosis). Androgen-independent human prostate cancer cells activate a genetic program of apoptotic cell death in response to exposure to ionizing radiation, in a dose-dependent fashion (Sklar, 1993). Results from animal models suggest that it is better to achieve maximal androgen suppression before starting radiation treatment however, in humans this may be sequence and volume dependent as well (Roach M, 2012; Zietman, 2000).
Radiation tolerance levels have been determined for nearby normal tissues that are likely to be affected during conventional fractionated radiotherapy treatment of tumors arising from the urinary tract. The term conventional fractionation generally refers to the delivery of a single daily dose of 180 cGy (1.8 Gy) to 200 cGy (2.0 Gy). When used alone, cumulative doses of at least 65 Gy are necessary for local control of gross disease (adenocarcinomas, transitional cell carcinomas [TCCs], and squamous cell carcinomas) arising from the prostate, bladder, urethra, or ureters. When used prophylactically for presumed microscopic disease (lymph nodes) or postoperatively, doses of 45–50 Gy are generally sufficient. For testicular seminomas, doses of 25 Gy are usually adequate.
Total dose, dose per fraction, and volume of normal tissue irradiated are the major risk factors for radiation-induced complications. The presence of several comorbid conditions such as previous surgery, diabetes, inflammatory bowel diseases, or old age is also associated with an increased risk of radiation-induced complications. Accurate estimates of the “true tolerance” of surrounding normal tissues have been hampered until recently by our inability to reconstruct the actual relationship between normal tissue doses and volumes in three dimensions. Early reports assumed that organ movement and day-to-day treatment setup error had an insignificant impact on the doses of radiation delivered to surrounding normal tissues. Studies have demonstrated that these assumptions are inaccurate and probably result in an underestimate of the true tolerance of surrounding normal tissues to radiation (Langen and Jones, 2001).
Dose per fraction, the total dose, volume, and overall treatment time are all critical determinants of chronic genitourinary toxicity. The linear quadratic equation (L-Q equation) has been adopted by many clinical investigators as the most useful model for comparing various doses and fractionation schemes (Fowler et al, 2001). This equation can be written as follows:
Effect (E) = n (αd+βd)
where
d= Dose per fraction
α = Nonrepairable effects
β = Repairable effects
n= Number of identical fractions
For comparing two different fractionation schemes, assuming a similar overall treatment time, the L-Q equation also can be written as follows:
D2/D1= (1 +d1β/α)/(1 +d2β/α)
where
D= Total dose and D1=n1d1
d= Dose per fraction
For most clinical circumstances, it is assumed that the α/β ratio for late-reacting normal tissues such as the bladder or rectum is 3. For early-responding normal tissues and for tumor, it is assumed that the α/β ratio is 10.
Radiobiologic modeling using the α/β ratios as described previously has been used to develop “altered fractionation schedules” to improve the therapeutic ratio between efficacy and toxicity. Accelerated hyperfractionation was until recently the most frequently used altered fractionation schedule. With accelerated hyperfractionation, more than one treatment is given per day with a minimum of 6 hours between treatments, using a decreased dose per fraction. Since most radiation-induced damage is repaired within 6 hours, the use of multiple treatments per day in theory should allow greater doses of radiation to be given over a shorter period of time, reducing the opportunity for tumor repopulation. Using α/β modeling, the late effects predicted using 1.2 Gy fractions twice daily (separated by 6 hours) to 69.6 Gy would be expected to be equivalent to those using conventional fractionation to 58 Gy. In contrast, early-responding tissues such as the epithelium of the bladder would be expected to respond as though they had been treated with a dose of 65 Gy using conventional fractionation. This model predicts an increase in acute effects, including tumor response, but a decrease in late effects, such as fibrosis.
Hypofractionation involves the use of larger than conventional fraction sizes. Such an approach results in nearly a 10% reduction in the total dose to the tumor without sparing late complications (assuming that the values chosen for the α/β ratio are correct for normal tissues and for tumors) (Fowler et al, 2001). Several recent investigators have argued that the α/β ratio for prostate cancer is much lower than previously thought and that this formed a strong rationale for the use of high dose rate (HDR) to treat prostate cancer (Brenner and Hall, 1999; Fowler et al, 2001; King and Fowler, 2001). For patients being treated with external-beam radiotherapy (EBRT), a growing number of investigators are studying the use of hypofractionated radiotherapy in an attempt to shorten overall time and cost, but to date, only one randomized trial suggests that cancer control rates might be better (Arcangeli et al, 2010, 2011; Lee, 2009; McCammon et al, 2009; Ritter et al, 2009). Some studies show no advantages, while others suggest there may be advantages to the use of hypofractionation (Arcangeli et al, 2010, 2011; Miles, 2008; Speight, 2005). Because of day-to-day setup variation, some type of online monitoring is advisable to ensure that this can be carried out safely.
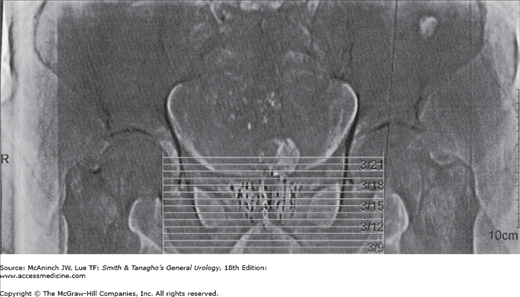
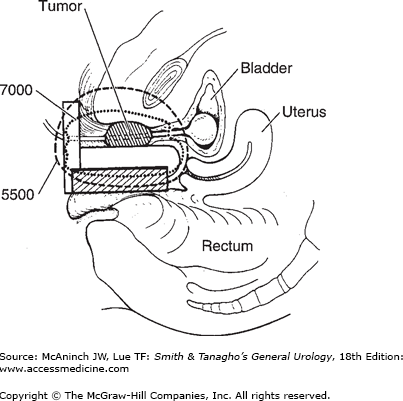
The term brachytherapy refers to a treatment technique that places radioactive sources in close proximity to or directly into the tumor. Brachytherapy can be classified as either interstitial or intracavity. Interstitial brachytherapy involves the placement of radioactive needles, afterloaded needles or catheters, or radioactive seeds directly into the prostate, bladder, penis, or periurethral soft tissues. Intracavitary brachytherapy includes placement of radioactive catheters into a lumen or orifice, such as in the urethra, to treat urethral and penile tumors. Permanent implants involve the use of radioactive seeds that are left in the patient. Nowadays, temporary implants generally involve the use of needles or catheters that act as conduits for delivering radiation from high-activity seeds attached to a long wire. Most modern temporary implants are delivered using HDR brachytherapy systems to deliver moderately high doses of radiation over a relatively short period of time (minutes). HDR brachytherapy is usually delivered over two or more treatment sessions to reduce the risk of late complications. Low dose rate brachytherapy is usually delivered in a continuous fashion over days to weeks via temporary or permanent implants, respectively. Figure 26–1 depicts an example of a transrectal ultrasound–based iodine-125 permanent interstitial implant of the prostate. Figure 26–2 shows an example of intracavitary brachytherapy for a urethral tumor in a female patient.
Specific Urologic Sites
Few urologists are aware that radiotherapy was first used to successfully treat prostate cancer 100 years ago and that it was not until 1949 that Memmelaar first reported the successful use of the retropubic approach—the radical RRP—for treatment of prostate cancer (Memmelaar, 1949). In the 1950s and 1960s, it was believed that radiotherapy provided comparable results with less morbidity. However, in 1979, Walsh and Donker demonstrated the potential value of the RRP to preserve nerves (Elder, 1982). Radical perineal prostatectomies became less fashionable in the 1980s after Walsh and others reported improvements in the RRP that promised to reduce blood loss, minimize the risk of incontinence, and preserve potency (Reiner, 1979). The comparative effectiveness of RRP compared with radiotherapy is very controversial in part because of major selection biases toward more favorable patients undergoing surgery and major advances in the accuracy and doses of radiation that can now be delivered (Giordano et al, 2008; Roach et al, 2008; Speight, 2007). Although some retrospective studies suggest better outcomes with surgery, inherent biases shed doubt on the validity of these conclusions (Roach et al, 2008).
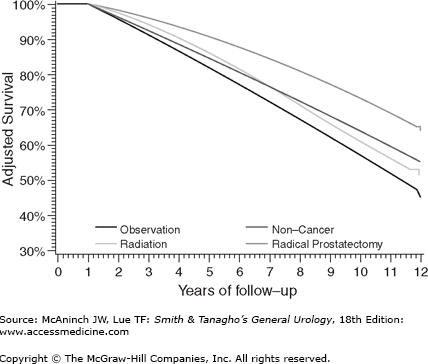
For example, Giordano et al set to systematically evaluate the effect of selection biases in observational studies of treatment effectiveness in men with localized prostate cancer (Giordano et al, 2008). They identified men from the Medicare-linked database—Surveillance, Epidemiology, and End Results. They noted that controlling for comorbidity, extent of disease, and other characteristics by multivariate analyses or by propensity analyses had remarkably small impact on these improbable results. They concluded that observational studies of treatment outcomes should be viewed with caution. As is shown in Figure 26–3, they noted that men treated by radical prostatectomy had a better adjusted survival rate than men without prostate cancer suggesting selection biases for favorable outcomes for these men compared with those treated with radiotherapy. Prostate-specific antigen (PSA) control rates appear to be very similar between contemporary high dose radiation and surgical series (Jabbari et al, 2010; Kupelian, 2004; Stone et al, 2009).
Figure 26–3.
As is shown, men treated by radical prostatectomy had a better adjusted survival rate than men without prostate cancer suggesting selection biases for favorable outcomes for these men compared with those treated with radiotherapy. (From Giordano SH et al: Limits of observational data in determining outcomes from cancer therapy. Cancer 2008;112:2456–2466.)
Conventional external beam radiation therapy (EBRT) has been used in the United States for treating prostate cancer since the late 1960s through the late 1980s (Bagshaw et al, 1988). The placement of the treatment fields was based on bony anatomic landmarks. It is now known that this technique resulted in inadequate coverage of the target volume in nearly one-third of patients. Computed tomography has improved our ability to localize and reconstruct pelvic anatomy, allowing more accurate design of treatment portals.
Based on these studies, the local control rate following EBRT has been estimated to be between 70% and 90%. The end points of these studies were based primarily on a clinical assessment of “local control.” These end points are now known to underestimate the true incidence of local failures because of the presence of occult cancer in clinically controlled patients. Many patients whose disease was once thought to be controlled locally, based on physical examination, do in fact have persistent disease when assessed by PSA or biopsy, or both (Crook et al, 2000). Although the posttreatment PSA is more sensitive, it is a less specific end point for treatment failure than is biopsy, because distant failures can contribute to a rising level. PSA has been long recognized as an acceptable end point for the assessment of disease status, but over the last few years, its value and limitations after radiotherapy have become much cleared (Roach et al, 2003, 2006a, 2006b, 2007; Rodrigues et al, 2011).
The standard or so-called Phoenix Definition for a recurrence is reached when a patient’s PSA rises by more than 2 ng/mL above the nadir (lowest) posttreatment value (Abramowitz et al, 2008). Despite its use to determine biochemical failure, PSA is not the most important predictor of survival being less important than Gleason score or T-stage even if very elevated to an ultra-high levels (eg, ≥50 ng/mL). In contrast, the importance of GS and stage has been well demonstrated by the long-term results of Phase III randomized trials conducted by the Radiation Therapy Oncology Group (RTOG) (Roach et al, 2000). In a multivariate analysis, Gleason score, clinical stage, and pathologic node status were correlated with overall and cause-specific survival. The use of this type of risk stratification system to predict overall survival has been validated using contemporary patients (Roach et al, 2007). A benefit of the RTOG risk groups is their use in defining for whom and how long hormonal therapy should be used. Numerous other risk stratification schemes are currently in popular use by radiation oncologists in the management of prostate cancer, but the greatest degree of consensus is probably the groupings recommended by the National Comprehensive Cancer Network (NCCN) (Mohler et al, 2010).
Many of the technical challenges with delivering accurate and high dose radiation have been addressed by the incorporation of (1) computed tomography–assisted localization and reconstruction of the pelvic anatomy in the early 1990s resulting in 3-DCRT; (2) computer optimization algorithms to improve dose conformality in the mid-1990s to create IMRT; (3) online imaging to create so-called image-guided IMRT or IGRT as the new standard of care for treating clinically localized prostate cancer (Boyer et al, 2001).
The most common type of IGRT used involves the use of intraprostatic markers (usually by urologist) that can be used to adjust for setup (positioning) errors and organ displacement immediately prior to each treatment (Shinohara, 2008). More sophisticated technologies have been developed but have not been proven to render better results and are more expensive (Langen et al, 2003; Willoughby et al, 2006). Because IGRT allows more accurate administration of radiation to the target, less normal tissue receives radiation, and, therefore, less side effects are observed (Millender et al, 2004). This may in turn permit increased doses of radiation to be given and theoretically lead to improved local control.
Following the early Phase I–II studies conducted by the RTOG (9406), higher doses (>70 Gy) became widely accepted as standard among radiation oncologist (Michalski et al, 2009). To date, however, the use of higher doses has resulted only in improved rates of freedom from PSA failure (Dearnaley et al, 2005; Kuban et al, 2008; Peeters et al, 2006; Zietman et al, 2005). In contrast, the evidence for the use of androgen deprivation therapy (ADT) in conjunction with EBRT appears to be more compelling (Roach, 2007). RTOG 0815 is an ongoing Phase III trial attempting to determine whether ADT can be omitted when high doses of radiation are used. An earlier study conducted by the Medical Research Council (MRC) suggests that the use of ADT did not obviate the need for modest dose escalation (Dearnaley et al, 2007) (Table 26–1).
First author (year) source | Trial design | 5 y estimated control low vs high dose arm | T-Stages and PSA (Median)ch26tb1.fn1 | Impact of higher doses of radiation |
|---|---|---|---|---|
Pollack et al (2002) MD Anderson | 70 vs 78 Gy | 60/90% | T1–3 7.8 ng/mL | Improved PSA control rates and trend for reduced DM |
Sathya et al (2005) Ontario and Hamilton | 66 Gy EBRT vs 40 Gy EBRT + 35 Gy iridium 192 boost | ∼40/70% | T2 and T3 19 ng/mL | Better outcomes with iridium implant (higher biologic dose) |
Lukka et al (2005) NCI Canada | 66 Gy in 33 fractions vs 52.5 Gy in 20 fractions | 53/60% | T1c to T2c 10.5 ng/mL | Patients receiving lower doses did worse |
Zietman et al (2005) Proton Radiation Oncology Group | 70 Gy vs 79 GyE (protons) | 60/82% | T1–2, PSA 6.3 ng/mL | Improved PSA control rates, no difference in OS, CSS, DM |
Peeters et al (2006) The Netherlands | 68 vs 78 Gy | 64/74% | T1–4 10–20 ng/mL | Improved PSA control rates (not by Phoenix definition) no difference in OS, CSS, DM |
Dearnaley et al (2007) MRC RT01 | 64ch26tb1.fn2 vs 74 Gych26tb1.fn2 | 60/71% | T1–3 12.8 ng/mL | Improved PSA control rates, no difference in OS, CSS, DM |
Alternative forms of radiation for the treatment of prostate cancer grew in popularity in the late 1990s but plateaued with the development of IGRT and proton beam radiotherapy. The most common of these alternative forms of radiation is brachytherapy. The major theoretic advantages with this form of radiation are the ability to deliver a very high dose of radiation to a localized area with a decreased number of treatment visits. The use of modern-era imaging techniques for visualizing the placement of radioactive seeds has obviated the need for open surgical procedures. Transrectal ultrasound–guided closed techniques are currently the standard. Permanent implants involve the use of low dose rate (LDR) radiation delivered to a very higher total dose (eg, >100 Gy). Temporary implants generally involve a lower total dose but at a higher dose rate, hence the term “high dose rate” brachytherapy. An example of an ultrasound-based iodine-125 permanent seed implant of the prostate, performed at our institution, is shown in Figure 26–1.
The failure rates reported in several older studies (done in the late 1960s and 1970s) suggested that the permanent implants are less effective than EBRT. More recent series suggest that the results of permanent implants may be equal to or better than other treatments (Jabbari et al, 2010; Pickles et al, 2010).
At most centers, intermediate- and high-risk patients are treated with a combination of EBRT and interstitial implant with or without hormonal therapy. Some clinicians routinely add EBRT for all patients undergoing permanent seed implants (Critz et al, 2000). However, most brachytherapists agree that low-risk patients can be equally well treated without the additional cost or morbidity of EBRT, while patients with intermediate-risk disease usually receive EBRT (Frank et al, 2007; Merrick et al, 2006; Nag et al, 1999). Although proponents of prostate brachytherapy commonly believe that the morbidity associated with interstitial brachytherapy is less than that associated with 3-DCRT, prospective studies using validated quality-of-life instruments suggest that acute morbidity is greater with permanent implants, whereas long-term morbidity tends to be similar (Sanda et al, 2008).
Temporary implants have the advantage of decreasing radiation exposure to hospital personnel and greater flexibility because of the ability to compensate for less than optimal needle placement. Temporary implants tend to be used for patients with more advanced disease in part because they are usually combined with EBRT and because HDR can be used to cover disease that is thought to be outside the gland. Iridium-192 is the only widely used isotope for temporary prostate implants. Based on the available data, in the hands of experts, it appears that HDR represents an excellent treatment option.
Particle beam radiation is an alternative form of EBRT. This class of radiation involves the use of heavy particles (eg, neutrons), charged particles (eg, protons), or heavy-charged particles (eg, carbon). The theoretic advantage of proton-based radiotherapy is the potential for a more conformal dose distribution. Two prospective randomized trials have been completed to date. The first showed a significant improvement in local control in patients with high-grade tumors but no improvement in disease-free, relapse-free, or overall survival (Shipley et al, 1995). There was no benefit to other subsets of patients, and the 5-year actuarial rates of rectal bleeding were significantly higher (p= 0.002) with mixed beam treatment. The second trial essentially confirmed the dose response findings of earlier x-ray–based dose escalation trials with better PSA control for men treated to 79 Gy than those treated to 70 Gy (Zietman et al, 2005).
The attractiveness of heavy particles such as neutron (or carbon) based radiotherapy relates to the relative lack of oxygen dependence. Heavy-charged particles (such as carbon) are thought to have the advantages of both neutrons and protons. Early studies using this technology have been encouraging, but the series are small, follow-up is relatively short, and this equipment has limited availability (Tsuji et al, 2005). Longer follow-up studies will be required to assess the impact of these alternative types of radiation on long-term survival.
Regarding hormonal therapy and radiotherapy, there currently exists no uniform consensus as to who should receive hormonal therapy and how long it should last. However, the best evidence suggests that low-risk patients do not benefit, intermediate-risk patients benefit from short-term hormonal therapy, and high-risk patients benefit from long-term hormonal therapy (Roach et al, 2010). The standard for “short-term” hormonal therapy consists of combined androgen blockade using a luteinizing hormone-releasing hormone agent and an antiandrogen 2 months before (neoadjuvant) and 2 months during radiotherapy with or without 2 additional months of ADT for a total of 4–6 months of combined ADT. Patients receiving hormonal therapy appear to benefit with or without whole-pelvic radiotherapy. Most patients with high-risk disease (T3 GS = 7–10 or GS = 8–10) should also receive long-term adjuvant ADT with a luteinizing hormone-releasing hormone agonist for 2–3 years or longer in selected patients (Roach et al, 2010). Tables 26–2 and 26–3 summarize the major Phase III trials including short- and long-term ADT and EBRT, respectively. ADT alone is not as effective as ADT combined with EBRT (Mason et al, 2010; Widmark et al, 2009).
First author (year) source | Pts | Arms | Benefit |
|---|---|---|---|
Roach et al (2008) RTOG 8610 | 456 pts, T2–4 | 44 WP −> 66–70 Gy +/− 4 mo NHT (2 mo before and 2 mo concurrent) | Improved 10-y CSS, reduced DM: 47 −> 35% |
D’Amico et al (2004) Dana Farber | 206 pts | 45 Gy to P& SV−> boost P to 70 Gy +/− 6 mo ADT (2 mo before, during, and after) | 8-yr OS: 61 −> 74% for all pts, mainly those with no or minimal comorbidities 5-y: bF: 45 −> 21% CSS: 94 −> 100% FF salvage HT: 57 −> 82% |
Denham et al (2005) TTROG | 818 pts, T2b-T4 | 66 Gy to prostate & SV 0 vs 3 mo vs 6 mo NHT (2 & 5 mos before RT) | Compared 0 vs 3 vs 6 mo ADT: bFFS: 38 vs 52 vs 56% 6 vs 0 mo improved DM and PCSS |
Crook et al (2004) Princess Margret | 378 pts, T1c-T4 | 66–67 Gy to prostate (+WP 45–46 Gy if risk of LN >15%) 3 mo vs 8 mo nADT | No difference in 5-y FFF or OS. For high-risk pts only, DFS improved in the 8-month arm (71% vs 42%, p= 0.01) |
Laverdiere (2004) Quebec | 481 pts, T2–3N0 | 64 Gy to prostate-only: Study 1: 0 vs 3 mo ADT vs 10 mo ADT Study 2: 5 mo ADT vs 10 mo ADT | 7 y bPFS: 42 −> 67% with 3–10 mo HT vs no HT No diff in bPFS with 5 vs 10 mo ADT in 2nd study |
Study | Pts | Arms | Benefit |
|---|---|---|---|
RTOG 8531 (Pilepich et al, 2005) | 945 pts, cT3, pT3, or LN+ | 65–70 Gy +/− indefinite AHT the last week of RT | Improved 10 y OS |
Bolla et al (2002) EORTC 22863 | 415 pts, T3–4 or T1–2 G ≥ 7 | 70 Gy +/− 3 y AHT starting on first day of RT | Improved 10 y OS and CSS |
Hanks et al (2003) RTOG 9202 | 1554 pts, T2c-T4 PSA <150 ng/mL | 65–70 Gy + 4 mo NHT +/− 2 y AHT | 10 y OS: 52 −> 54% overall survival advantage for GS 8–10: 32 −> 45% CSS and DM advantage |
Bolla et al (2009) EORTC 22961 | 970 pts, T2c-T4N0/+ or T1c-2bN+ | 70 Gy; 6 mo ADT vs 3 y AHT | 3 y LHRH improved 5 y OS; prostate cancer mortality: 4.7% vs 3.2%, no difference in fatal cardiac events (3–4%) |
The objectives of local regional postoperative radiotherapy may include attempting to eliminate microscopic residual tumor in the surgical bed, regional periprostatic tissues, and regional lymph nodes. As such, there are several potential indications for the use of adjuvant radiation, including (1) positive surgical margins, (2) seminal vesicle involvement, (3) lymph node involvement, (4) extracapsular extension, (5) increasing PSA, and (6) biopsy-proven recurrence. The presence of any of these variables is associated with a higher incidence of local recurrence. Radiotherapy may be administered prior to evidence of a recurrence or so-called adjuvant treatment or after documented failure (usually based on a detectable or rising PSA) or so-called salvage treatment. Adjuvant EBRT reduces the incidence of local recurrence in patients with postsurgical microscopic residual tumor after radical prostatectomy. The major Phase III trials have consistently shown a delay in the risk of biochemical and clinical failure and the trial with the longest follow-up shows a reduction in the mortality associated with adjuvant EBRT compared with delayed treatment (Bolla et al, 2002; Thompson et al, 2009; Wiegel et al, 2009). There is a common impression among some urologists that the risk of complications outweighs the benefits of adjuvant radiotherapy, but this impression is probably due to the consequence of older treatment techniques. More contemporary series suggest that, with modern equipment and 3-D planning, the incidence of complications is relatively low (Moinpour et al, 2008; Pinkawa et al, 2008; Thompson, 2009).
The findings from these trials are consistent with those of retrospective studies that suggest that patients who are treated before clinically manifesting a local recurrence appear to have improved disease-free survival, time to distant metastasis, and freedom from biochemical relapse compared with patients undergoing salvage treatment (Nudell et al, 1999; Valicenti et al, 1999). Only 50% of such patients are successfully treated for biopsy-proven recurrence at 3 years (Rogers et al, 1998). Salvage radiotherapy, however, is the only curative option in men experiencing PSA failure after surgery. A nomogram reported by Stephenson et al provides a reasonable estimate of the overall effectiveness of men undergoing salvage EBRT for most patients (Stephenson et al, 2007). Overall only 25% are long-term disease free with some subsets doing substantially better. For example, those with a pre-EBRT PSA <0.5 ng/mL, a long doubling time (>6 years), and positive margins but negative seminal vesicles and lymph nodes may achieve progression free rate of up to 80% at 6 years when treated with conventional dose radiation and ADT.
Although definitive proof from a prospective randomized trial is lacking, it seems reasonable to assume that patients who have no other disease away from the pelvis would benefit from adjuvant treatment. Retrospective studies suggest that pelvic lymph nodal treatment may improve outcomes compared with ADT alone (Da Pozzo et al, 2009; Spiotto et al, 2007). The RTOG is currently conducting a Phase III trial addressing this question (RTOG 0534).
Patients experiencing local recurrences after EBRT or brachytherapy may be candidates for salvage radiotherapy (Aaronson et al, 2009; Lee et al, 2007). Unfortunately, the complication rate is quite high in some series (Nguyen et al, 2009). The RTOG is conducting a Phase I–II trial (RTOG 0526) of brachytherapy salvage of EBRT failures as well.
Most patients experience urinary frequency and dysuria during the course of their treatment. In patients receiving whole-pelvic irradiation, mild diarrhea may develop, but moderate to severe late complications are similar (Pinkawa et al, 2008; Pinkawa et al, 2011). Mild, self-limited rectal bleeding occurs in approximately 10% of patients and is dose and volume related. Urinary incontinence is usually associated with a history of a prior transurethral resection of the prostate. Hematuria and ureteral strictures occur in <2–10% of patients and are usually mild and self-limited. Following conventional EBRT Fecal incontinence was uncommon, but rectal urgency due to reduction in rectal dispensability occurred in 10% of patients (Lukka et al, 2005). Loss of erectile function is the most worrisome, the most common, long-term complication of radiotherapy. Impotence is reported in 35–40% of patients who were potent before treatment and maybe critically dependent on the dose of radiation received by the bulb of the penis (Roach et al, 2010). One rather large prospective study concluded that sexual dysfunction may be slightly lower after EBRT than after brachytherapy, but both had a lower impact on function than radical prostatectomy or cryosurgery (Sanda et al, 2008). However, most patients experience a decrease in the frequency and quality of intercourse, and most note a decrease in the volume of ejaculate. Potency diminishes further with time owing to aging and late radiation–induced normal tissue injury.
Acute urinary toxicity associated with brachytherapy is more common and longer lasting than that seen with 3-DCRT. The incidence of strictures is also higher. Acute obstruction occurs in 2–20% of patients. Although incontinence is quite uncommon following radiotherapy, it can occur in up to 50% of patients if they have had a previous transurethral resection of the prostate. The frequency of rectal toxicity following brachytherapy is generally believed to be less than that with 3DCRT (Sanda et al, 2008).
Nonprostate Genitourinary Cancers
Urothelial cancers (UCs) can occur along the entirety of the urinary tract, from the kidney to the urethra. Most occur in the bladder, though up to 5% of UC occur in the upper urinary tract. The majority of these involve the renal pelvis (Munoz, 2000). The role for radiation therapy in the management of UC varies by site, ranging from a palliative and up-and-coming role, as in the management of renal cell cancers, to an established and joint role with surgery and chemotherapy, as in organ-sparing approaches for the management of muscle-invasive bladder cancer, to a primary role, as can be considered for the management of penile cancer. This section will review the common uses of EBRT and brachytherapy in the management of urinary tract malignancies.
In the absence of durable local control, the natural history of bladder cancer is that of progressive growth and invasion with the eventual development of distant metastases. At diagnosis, the majority of patients (85%) with TCC of the bladder have superficial mucosal lesions (Ta, T1); however, 70% of patients recur locally following transurethral resection of the bladder tumor (TURBT). About 50–65% of these patients will progress to muscle-invasive disease (Brake, 2000). The presence of TCC in situ is associated with a more aggressive natural history, with higher probability of recurrence and progression to muscle invasive disease (Wolf, 1994). The addition of intravesicle immunotherapy (Bacille Calmette-Guerin, BCG) or chemotherapy decreases the overall recurrence rate by approximately 30% compared with TURBT alone. Nonetheless, within the first 5 years, tumor progression is noted in 20–40% of patients despite this additional treatment (Smith, 1999; Soloway, 2002). The development of muscle invasion (T2–T4) is accompanied by a significant increase in the incidence of metastatic spread and cause-specific death. Unfortunately, more than half of the patients diagnosed with muscle-invasive TCC have disseminated disease, often occult, at diagnosis. Five-year survival rates of up to 60% are reported for early invasive lesions (T1/T2a, N0); however, rates fall to ≤40% for more advanced tumors (T2b/T4, N+). Late systemic disease recurrence, most frequently pulmonary metastases, with or without local recurrence accounts for the decline in survival (Stein, 2001; Dalbagni et al, 2001), emphasizing the importance of adjuvant cytotoxic chemotherapy in the management of TCC. Following decades of unsuccessful single and bimodality treatments, contemporary management utilizes combinations of cytotoxic chemotherapy, radiotherapy, and/or surgery in an attempt to improve survival and, if possible, achieve organ preservation.
EBRT has had no role in the management of in situ (Tis) or superficial (T1) bladder cancer. Investigators at the University of Erlangen, Germany, proposed a role for post-TURBT EBRT or EBRT with chemotherapy (EBRT/CT; cisplatin or carboplatin with 5-fluorouracil) for high-risk (T1G3, T1G1–2 associated with Tis, multifocality or tumor diameter >5 cm) or multiple recurrent superficial bladder cancers (Weiss, 2006). Eighty-eight percent of (121/137) patients treated with EBRT or EBRT/CT 4–6 weeks after initial TURBT were found to have a complete response (CR) at restaging TURBT. Patients not achieving a CR (16/137; 12%) were managed with immediate cystectomy. Five- and 10-year DSS and OS rates for patients with CR were 89% and 75% and 79% and 53%, respectively. When the evaluation was limited to patients with T1G3 tumors, 5- and 10-year DSS and OS rates were 80% and 64% and 71% and 47%, respectively. These rates are comparable with those seen in primary cystectomy series in T1 bladder cancer (Amling, 1994; Freeman, 1995; Malkowicz, 1990). Of note, patients receiving EBRT/CT had significantly higher 5-year DSS rates than patients treated with EBRT only. These findings are provocative; however, a randomized trial comparing EBRT or EBRT/CT with BCG will be necessary to fully investigate the utility of this organ-sparing approach. A Phase III randomized trial by Harland et al reported that adjuvant EBRT provided no benefit over observation alone for time to progression, progression-free survival, or OS for T1G3 bladder tumors (Harland et al, 2007).
The primary use of EBRT has been in muscle-invasive TCC; however, many oncologists have felt that the role for radiotherapy in the management of TCC has been limited. Surgical and medical oncologists typically recommend EBRT only for those patients who are medically unfit for or refuse cystectomy, or as palliation for locally advanced, unresectable tumors. Radical cystectomy remains the “gold standard” for the management of recurrent superficial and primary muscle-invasive TCC in the United States, despite the absence of robust evidence supporting its superiority. In fact, the “optimum” management approach remains undetermined.
In earlier studies, neither radiotherapy monotherapy nor precystectomy radiotherapy has shown DSS or OS benefits versus radical cystectomy (Huncharek, 1998). However, most of these studies had small sample sizes, compared pathologically and clinically staged patients, and used inadequate radiotherapy techniques by current standards. Radiotherapy monotherapy yields poorer local control rates but comparable 5-year survival rates to radical cystectomy. For muscle-invasive TCC, three of four randomized trials comparing EBRT (≤50 Gy) plus immediate cystectomy versus primary EBRT (60 Gy) and delayed (salvage) cystectomy demonstrated equivalent long-term survival rates with either treatment; only one trial demonstrated a significant benefit associated with immediate cystectomy (Bloom, 1982; Miller, 1977; Sell, 1991). In addition, no significant difference in 5- and 10-year survival rates or rates of development of distant metastases is seen with delayed or salvage cystectomy (Horowich, 1995; Petrovich, 2001). The use of combined modality treatments to achieve organ preservation without compromising treatment outcome has become a management approach of choice for many malignancies, including breast, esophageal, laryngeal, and anorectal cancers. Demonstration of equivalent outcomes with salvage surgery has made organ preservation a reasonable and appropriate treatment choice for some patients with muscle-invasive TCC.
Combined Modality Management of Muscle-Invasive Bladder Cancer (Transurethral Bladder Resection, Chemotherapy, and EBRT) and Organ Preservation
Several prospective randomized trials evaluating combined modality therapy for bladder preservation have been completed. In general, each of the trials has followed a common bladder-preservation algorithm including maximal TURBT, followed by induction chemoradiation, and an assessment of treatment response. Individuals with clinically CR continued with bladder-sparing therapy; all others were recommended for extirpative surgery. Completeness of TURBT (visibly complete vs not visibly complete) is associated with significantly lower salvage cystectomy rates.
The key features of the contemporary bladder-sparing trials are summarized in Table 26–4.
Series (y) | Induction treatmentch26tb4.fn1 | CR Ratech26tb1.fn2 | 5-y OS |
|---|---|---|---|
Shipley: RTOG 85–12 (1987) |