CHAPTER 28 Protein-Losing Gastroenteropathy
Drs. Karen Kim and Thomas Brasitus contributed to previous versions of this chapter.
DEFINITION AND NORMAL PHYSIOLOGY
In 1947, Maimon and colleagues postulated that fluid emanating from the large gastric folds in patients with Ménétrier’s disease was rich in protein. In 1949, Albright and colleagues discovered, using intravenous infusions of albumin, that hypoproteinemia resulted from excessive catabolism of albumin rather than decreased albumin synthesis.1 By 1956, Kimbel and colleagues demonstrated an increase in gastric albumin production in patients with chronic gastritis; a year later, Citrin and colleagues2 were able to show that the GI tract was the actual site of excess protein loss in patients with Ménétrier’s disease. They showed that the excess loss of intravenously administered radioiodinated albumin could be explained by the appearance of labeled protein in the gastric secretions of such patients.
Subsequent research using 131I-labeled polyvinylpyrrolidone, 51Cr-labeled albumin, and other radiolabeled proteins, as well as immunologic methods measuring enteric loss of α1-antitrypsin (α1-AT), has further characterized the role of the GI tract in the metabolism of serum proteins. In fact, GI tract loss of albumin normally accounts for only 2% to 15% of the total body degradation of albumin, but in patients with severe protein-losing GI disorders, this enteric protein loss may extend to up to 60% of the total albumin pool.3–5
Under physiologic conditions, most endogenous proteins found in the lumen of the GI tract are derived from sloughed enterocytes and from pancreatic and biliary secretions.6 Studies of serum protein loss into the GI tract measured by various methods (e.g., 67Cu-ceruloplasmin, 51Cr-albumin, or α1-AT clearance) have shown that daily enteric loss of serum proteins accounts for less than 1% to 2% of the serum protein pool in healthy individuals, with enteric loss of albumin accounting for less than 10% of total albumin catabolism. In normal subjects, the total albumin pool is approximately 3.9 g/kg in women and 4.7 g/kg in men, with a half-life of 15 to 33 days and a rate of hepatic albumin synthesis of 0.15 g/kg/day, equaling the rate of albumin degradation.7 Excess proteins that enter the GI tract are metabolized by existing proteases much like other peptides, broken down to constituent amino acids, and then reabsorbed. In healthy individuals, GI losses play only a minor role in total protein metabolism, and serum protein levels reflect the balance between protein synthesis and metabolism. However, this balance can be altered markedly in patients with protein-losing gastroenteropathy.8
PATHOPHYSIOLOGY
Excessive plasma protein loss across the GI epithelium can result from several pathologic alterations of healthy mucosa. Mucosal injury can result in increased permeability to plasma proteins; mucosal erosions and ulcerations can result in the loss of an inflammatory, protein-rich exudate; and lymphatic obstruction or increased lymphatic hydrostatic pressure can result in direct leakage of lymph, which contains plasma proteins. Changes in vascular permeability can affect the concentration of serum proteins in the interstitial fluid, thereby influencing the amount of enteric mucosal protein loss.9 Hypoproteinemia seen in GI disorders can therefore be classified into three groups: (1) increased mucosal permeability to proteins as a result of cell damage or cell loss; (2) mucosal erosions or ulcerations; and (3) lymphatic obstruction.
Examining the pathogenesis of protein-losing gastroenteropathy, Bode and colleagues have suggested that the condition might be related to loss of heparan sulfate proteins that are normally present on the surface of intestinal epithelial cells.10,11 Heparan sulfate proteoglycans appear to affect the intestinal barrier by having large extracellular domains that bind to the plasma membrane, known as syndecans, or are attached to a membrane glycolipid, called a glypican.12 These syndecans are important in the maintenance of tight intercellular junctions (see Chapters 2 and 96).
A myriad of diseases are associated with protein-losing gastroenteropathy. These are listed in Table 28-1 and discussed in more detail elsewhere in this text.13–63
Table 28-1 Disorders Associated with Protein-Losing Gastroenteropathy
AIDS, acquired immunodeficiency syndrome.
Mice that were genetically altered to lack syndecans or other heparan sulfate proteins have alterations to the normal tight intercellular barrier, and leak protein via paracellular pathways into the intestinal lumen (Fig. 28-1). Moreover, treatment of such mice with proinflammatory cytokines such as tumor necrosis factor-α (TNF-α) or interferon-γ leads to significantly defective intercellular junctions and even greater protein loss into the intestine.10 The combination of a syndecan-deficient state and exposure to proinflammatory cytokines leads to even greater albumin flux and protein loss. Finally, reintroduction of heparin sulfate or other syndecans abolishes the protein loss into the lumen of the bowel.
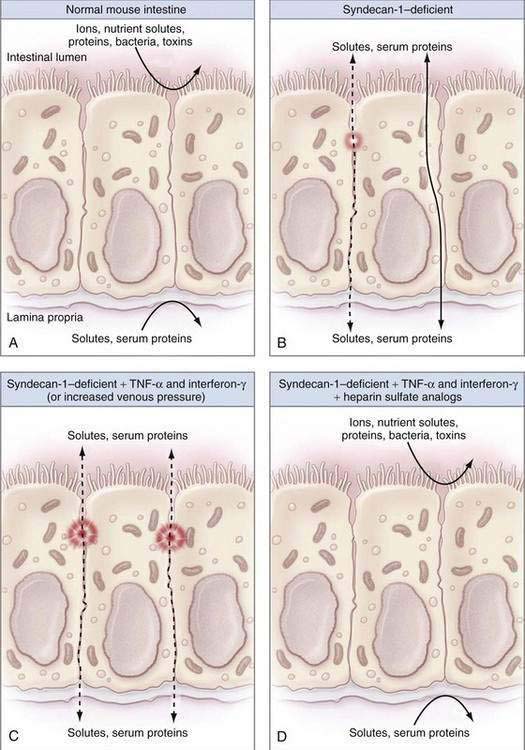
Figure 28-1. Diagrams illustrating the factors that contribute to intestinal integrity in the mouse. A, The normal mouse intestine makes an effective barrier against free diffusion of certain ions, nutrient solutes, proteins, bacteria, and toxins to separate the intestinal lumen (outside) from the lamina propria (inside) effectively. B, As noted by Bode and colleagues,10 syndecan-1–deficient mice have decreased intestinal barrier function as a result of defective intercellular junctions and increased paracellular leaks (dashed line) or increased transcellular protein transport (solid line). C, Syndecan-1–deficient mice that were treated with inflammatory cytokines such as tumor necrosis factor-α (TNF-α) and interferon-γ or surgically to increase their portal venous pressure have massively defective intercellular junctions and large intercellular protein leaks (dashed lines), consistent with protein-losing enteropathy. D, Infusions of heparin sulfate analogs completely reverse the intestinal barrier dysfunction seen in syndecan-1–deficient mice treated with inflammatory cytokines. See text for more details.
(From Lencer WI. Patching a leaky intestine. N Engl J Med 2008; 359:526-8, with permission.)
The loss of serum proteins in patients with protein-losing gastroenteropathy is independent of their molecular weight, and therefore the fraction of the intravascular pool degraded daily remains the same for various proteins, including albumin, immunoglobulin G (IgG), IgA, IgM, and ceruloplasmin.8 In contrast, patients with nephrotic syndrome preferentially lose low molecular weight proteins such as albumin. As proteins cross into the GI tract, synthesis of new proteins occurs in a compensatory fashion. Proteins that enter the GI tract are metabolized into constituent amino acids by gastric, pancreatic, and small intestinal enzymes, reabsorbed by specific transporters, and recirculated. When the rate of gastric or enteric protein loss, or both, exceeds the body’s capacity to synthesize new protein, hypoproteinemia develops.6 Hypoalbuminemia, for example, is common in protein-losing gastroenteropathy and results when there is an imbalance between hepatic albumin synthesis, which is limited and can increase only by 25%, and albumin loss, with reductions in the total body albumin pool and albumin half-life.9
Adaptive changes in endogenous protein catabolism may compensate for excessive enteric protein loss, resulting in unequal loss of specific proteins. For example, proteins such as insulin, clotting factors, and IgE have rapid catabolic turnover rates (short half-lives) and, as such, are relatively unaffected by GI losses, because rapid synthesis of these proteins ensues. On the other hand, proteins such as albumin and most gamma globulins, except IgE, are limited in their ability to respond to GI losses, so protein loss from the gut will be manifested by hypoproteinemia (hypoalbuminemia and hypoglobulinemia).8 Other factors also can contribute to the excessive enteric protein loss seen in various diseases. These include impaired hepatic protein synthesis and increased endogenous degradation of plasma proteins.
In addition to hypoproteinemia, protein-losing gastroenteropathy can result in reduced concentrations of other serum components, such as lipids, iron, and trace metals.8 Lymphatic obstruction can result in lymphocytopenia, with resultant alterations in cellular immunity.
CLINICAL MANIFESTATIONS
Hypoproteinemia and edema are the principal clinical manifestations of protein-losing gastroenteropathy. Most other clinical features reflect the underlying disease process and, as such, the clinical presentation of patients with protein-losing gastroenteropathy is varied (Table 28-2). Hypoproteinemia, the most common clinical sequela, is manifested by a decrease in serum levels of albumin, gamma globulins (IgG, IgA, and IgM, but not IgE), fibrinogen, lipoproteins, α1-AT, transferrin, and ceruloplasmin.8 Levels of rapid turnover proteins, such as retinal binding protein and prealbumin, are typically preserved, despite hypoproteinemia.64 Dependent edema is frequently a clinically significant issue, and results from diminished plasma oncotic pressure. Anasarca is rare in protein-losing gastroenteropathy. Unilateral edema, upper extremity edema, facial edema and macular edema (with reversible blindness), and bilateral retinal detachments have been seen as a consequence of intestinal lymphangiectasia.65 Despite a decrease in serum gamma globulin levels, increased susceptibility to infections is uncommon. Although clotting factors may be lost into the GI tract, coagulation status typically remains unaffected. Circulating levels of proteins that bind hormones, such as cortisol and thyroid-binding proteins, may be substantially decreased, but levels of circulating free hormones are not significantly altered.
Table 28-2 Clinical Manifestations of Protein-Losing Gastroenteropathy
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree








