Fig. 35.1
The anterior and posterior portions of the urethra are depicted
The posterior urethra is composed of the prostatic urethra and the membranous, or intermediate, portion of the urethra. The prostatic urethra is the first portion of the urethra leading to the intermediate portion which is enclosed by the sphincter urethrae muscle. While the membranous portion of the posterior urethra is most commonly injured secondary to pelvic fractures, the prostatic segment of the posterior urethra is susceptible to iatrogenic injury [2]. The majority of prostatic urethral injuries are secondary to prostatic resections such as transurethral prostatic resections (TURP) or radical prostatectomies. Figure 35.2 demonstrates the close relation between the prostatic urethra and rectum, which makes it susceptible to iatrogenic injury during pelvic surgery. The discussion within this chapter is limited to injuries of the prostatic urethra which can occur during colorectal surgeries.
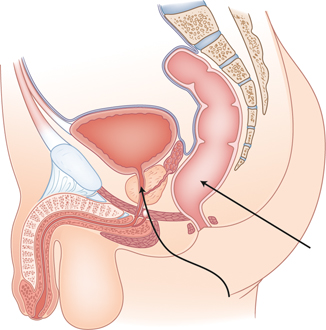

Fig. 35.2
Demonstration of the anatomy of the urethra in relation to the rectum. The straight arrow points to the rectum. The wavy arrow points to the prostatic urethra
Incidence
Colorectal operations at risk for injuring the urethra include proctectomies and abdominoperineal resections. Such injuries may occur during dissection for either benign or malignant conditions. This fact is not surprising considering that the anterior portion of the lower rectum is intimately associated with the posterior border of the prostate.
Types of Prostatic Urethral Injury
During pelvic surgery and dissection of the rectum, the prostatic urethra is prone to two different types of injury. Transection of the urethra during sharp dissection may lead to immediate urine leakage although transection with an energy device may temporarily occlude the lumen resulting in a delayed leak. Pelvic dissection with electrocautery may lead to ischemia of the urethra followed either by a stricture or a delayed leak.
Prevention
The key to preventing a prostatic urethral injury, or any urinary injury for that matter, is to be vigilant about the risk. This risk is increased in patients with bulky anterior rectal tumors, a history of radiation, and/or history of a prostatectomy, all of which may obliterate the rectoprostatic space. The inflammation and scar tissue in the former setting make it difficult to visualize and dissect in the plane immediately anterior to the rectoprostatic, or Denonvillier’s fascia. The large inflamed rectum in patients with proctitis and following an anastomotic leak also increases the risk of injury.
One technique to facilitate awareness of the location of the urethra is the placement of a large diameter Foley catheter. Alternatively, urethral sounds may be more easily palpated intraoperatively secondary to their rigidity. The wider diameter catheter can help the surgeon palpate the prostatic urethra especially when nearing it. However, it is important to not have an overwhelming sense of security with this technique as it may still be difficult to identify the location of the urethra in cases of previous radiation and/or prostatectomy. When reoperating on patients following a pelvic anastomotic leak or for a recurrent rectal carcinoma, special vigilance is needed.
Detection
Should the surgeon encounter the unfortunate situation of having injured the prostatic urethra, the ideal time for detecting the injury is during surgery. Early recognition may allow for synchronous repair and may potentially allow for a better repair without having to subsequently re-enter the pelvis.
Some surgeons advocate the routine use of indigo carmine or methylene blue during colorectal surgeries in which the urinary system is at risk. Therefore, if the surgeon is concerned about the prostatic urethra, such as in cases where bulky tumors or radiation have eliminated the normal planes, the anesthesiologist can administer 10 mL of indigo carmine or methylene blue intravenously with or without furosemide to expedite diuresis. Another intraoperative method for detecting injuries is to inject 10–20 ml of methylene blue via an angiocatheter placed in the urethra adjacent to the Foley catheter [1]. Again, any visualized extravasation of methylene blue would indicate injury to the urinary tract.
The surgeon may also encounter delayed recognition of urethral injury. Postoperative signs of urinary injury may include a rise in blood urea nitrogen (BUN) and creatinine levels secondary to urinary absorption from the peritoneal cavity and an increase in drain output, particularly if serous in quality. If an injury is suspected, the drain output can be sent to the laboratory for a creatinine level. A drain creatinine level in excess of the serum creatinine level indicates a urinary leak. A renal ultrasound may indicate dilatation of the more proximal urinary system or a distended bladder if the urethra is strictured. A computed tomography scan of the abdomen and pelvis with intravenous contrast may also be used to detect urinary injuries; however, the contrast must have reached the site of injury for extravasation to occur and will not indicate the specific site of injury.
A retrograde urethrogram can also be used to visualize urethral injuries, either intraoperatively or postoperatively. Rosenstein and Alsikafi have provided a detailed description the technical aspects of performing a retrograde urethrogram [3]. The patient is first positioned supine on the table, and then the left pelvis is elevated 30–45° from the table. The right thigh is bent at a right angle to the hip while the left leg is kept straight. A 14 French Foley catheter is then inserted into the tip of the penis and the Foley balloon distended with 2 ml of water; 30 ml of water-soluble contrast is then injected and a radiograph exposure taken at least near the end of the injection if not throughout the injection. This technique will allow appropriate visualization of the entire urethra and any leaks or strictures.
Management
A prostatic urethral injury detected at any time should lead to a urology consultation if available and primary repair at the initial operation. It is important to keep in mind that most data regarding the management and outcome of prostatic urethral injuries are based on traumatic urethral disruptions.
Intraoperative detection of a prostatic urethral injury will potentially allow for a primary repair if able to be performed in a tension-free manner [1]. The two ends of the urethra should be spatulated in such a repair to try to obviate subsequent anastomotic stricture. In patients who have undergone pelvic radiation, it may be prudent to reinforce the repair with omentum, a local tissue flap, and/or a biologic mesh [1]. In cases of significant loss of urethra, urethral reconstruction has been described using a pedicled gracilis flap [4]. During intraoperative repair of the urethra, if there is difficulty in identifying the more proximal urethra after a complete transection, a urethral sound may be placed through either a suprapubic location or by creating an anterior cystotomy.
Prostatic urethral injuries that are detected postoperatively are more difficult to manage. Unless a patient is within the first few postoperative days, there will likely be significant adhesions in the pelvis making any immediate surgical approach more difficult. Experience with traumatic posterior urethral injuries indicates that primary urethral realignment results in lower rates of fibrotic defects compared to primary bladder drainage with plans for delayed urethroplasty; however, the erectile dysfunction and urinary incontinence rates were higher [5–7]. Other authors have reported lower rates of erectile dysfunction and urinary incontinence following surgical realignment [8–10]. Primary urethral realignment can be performed either surgically, transabdominal or transperineal, or endoscopically. A urethral stricture, either from cautery injury or following initial management of the injury with primary urethral realignment, may be amenable to bulboprostatic anastomotic urethroplasty via either an abdominoperineal or transperineal approach [11–13]. It is important to keep in mind that some degree of the complications described with the various techniques may be related to the traumatic mechanism of injury.
The key to managing any urinary injury is to allow adequate drainage of the urinary system. A bladder catheter should be kept in place across the urethral injury postoperatively to keep the bladder decompressed. Some authors also advocate placing a suprapubic catheter in addition to the bladder catheter to ensure appropriate drainage should one mechanism fail. If, however, the urethral injury is postoperatively detected, a suprapubic catheter should be placed for drainage.
Additionally, drains placed adjacent to any of the above-discussed repairs will enable detection of urinary extravasation and, more importantly, help ensure adequate drainage should a urine leak or a fistula develop.
Delayed Rectourethral Fistula
A delayed urethral injury may also present as a rectourethral fistula. These patients may present with pneumaturia, fecaluria, urine draining through the rectum, or recurrent urinary tract infections. Different modes of treatment exist in the treatment of delayed iatrogenic rectourethral fistulas including transperineal, transanal, transsphincteric, and transabdominal approaches [14].
Transperineal repairs usually involve closure of the rectum and/or closure of the urethral opening. These outcomes are improved by interposing muscle between the rectal and urethral repairs, especially if the pelvis was previously irradiated. The gracilis muscle and dartos muscle interposition flaps are well described in the treatment of rectourethral fistulas [15–18]. The preference of the authors is to perform gracilis interposition flaps for pelvic fistula disease specifically using the transperineal approach because it provides great access to the fistula, brings a large piece of viable muscle to help buttress the fistula repair, and minimally affects the donor extremity with mild numbness being the main side effect [16, 19–21]. The gracilis muscle is a large muscle dependent on one main neurovascular bundle at its origin, which makes it versatile. All patients should undergo fecal diversion either prior to or occasionally at the time of graciloplasty. The creation of an ileostomy or colostomy should facilitate healing by reducing fecal contamination. Maintenance of an indwelling Foley catheter and occasionally also a suprapubic catheter throughout the duration of treatment is mandatory. The objective is to prevent both urine and stool from entering the area of the repair.
The technical aspects of performing a graciloplasty have been well described [19]. The gracilis muscle is harvested from the patient in the Lloyd-Davies position. A 3–4-cm incision is first made in the distal medial thigh, staying posterior to the saphenous vein. Dissection is carried down onto the gracilis muscle after which its tendon is encircled with either a red rubber catheter or a penrose drain. A second small incision is then made on the proximal thigh about four fingerbreadths distal to the pubic tubercle where the gracilis muscle is again identified and encircled. The surgeon then bluntly dissects through the space superficial to the gracilis muscle to create a tunnel connecting both incisions. The gracilis tendon is then divided from behind the medial condyle after which a laparoscopic energy device is used to circumferentially mobilize the muscle up to its neurovascular bundle 10 cm from the pubic tubercle. Throughout this procedure, it is important for the anesthesia team to avoid any paralytics so that the location of the neurovascular pedicle can be confirmed by stimulating the nerve. A tunnel is then created from the upper thigh incision to the site of the planned perineal incision. The thigh incisions are closed over a drain. The patient is then routinely repositioned into the prone jackknife position to optimize exposure. A 5-cm circumanal perineal incision is then made through the perineal body and carried proximally at least 2 cm above the fistula in healthy tissue. The edges of the fistula tract are then resected. While the rectal defect is always closed with an advancement flap, the urethral defect is almost always left open depending on the fistula size and on the condition of the surrounding tissues; very small urethral defects surrounded by healthy pliable tissue may occasionally be primarily repaired. A series of bilateral 2.0 prolene sutures are placed from the apex to the distal aspect of the dissected space, after which the sutures are passed through the gracilis muscle, interposing it between the rectum and urethra (Figs. 35.3, 35.4 and 35.5). A closed suction drain is also left under the perineal incision. Later, the patient is placed in an adduction splint prior to reversal of general anesthesia. Postoperatively, the patient is on bed rest with an adductor splint for 3 days, intravenous antibiotics for 3 days after which oral antibiotics are started, and a bladder catheter for 6–8 weeks. Successful fistula closure is verified 6 weeks following surgery with a water-soluble contrast enema, a retrograde urethrogram, cystoscopy, and examination under anesthesia. Stoma closure is generally performed 12 weeks after the surgery, at which time the Foley catheter is also removed.
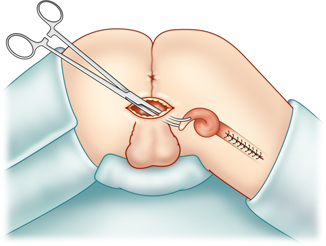
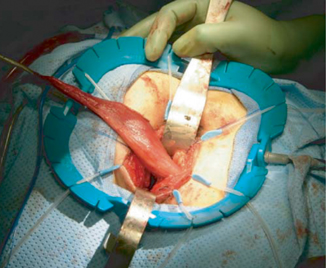
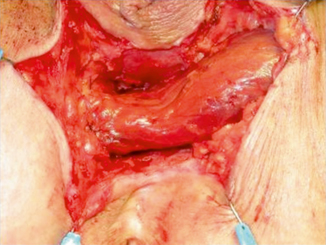

Fig. 35.3
The patient is in the prone jackknife position. The gracilis muscle is grasped and about to be pulled into the perineal incision

Fig. 35.4
The gracilis muscle after being pulled through the perineal incision. (With permission from Zmora et al. [15] © by Lippincott Williams & Wilkins)

Fig. 35.5
The gracilis muscle being interposed between the rectum and urethra. (With permission from:Zmora et al. [15] © by Lippincott Williams & Wilkins)
We reviewed our results with gracilis interposition flaps for rectourethral, rectovaginal, and pouch-vaginal fistulas [16]. Our results included 53 patients from 1995 to 2007 including 36 males with a rectourethral fistula and 17 females, 15 of whom had a rectovaginal fistula. Thirty of the 36 males had undergone treatment for prostate carcinoma. Five males required a second gracilis interposition flap for the following reasons: failure after an initial gracilis flap, intraoperative muscle necrosis, perineal sepsis requiring debridement, and persistent fistula. Only one of those males had a persistent fistula after two gracilis flaps; however, he eventually healed his fistula after a transanal rectal advancement flap. There were 23 complications in 17 patients: perineal wound infection, urethral stricture, prolonged perineal wound drainage, fever, urinary retention, urinary tract infection, perineal bleeding, penile cellulitis, deep venous thrombosis, thigh hematoma, thigh pain/numbness, and fecal incontinence following stoma reversal. Thus, our success rates were 78 % after initial graciloplasty and 97 % after secondary procedures. Table 35.1 includes a review of the success rates with graciloplasty in treating rectourethral fistulas.




Table 35.1
Review of gracilis interposition for repair of rectourethral fistula
< div class='tao-gold-member'>
Only gold members can continue reading. Log In or Register to continue
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree








