Grade A
Grade B
Grade C
Specific treatment
Not required
Fresh frozen plasma
Transfer to intensive care unit
Albumin
Circulatory support (vasoactive drugs)
Daily diuretics
Hemodialysis
Noninvasive ventilation
Intubation and mechanical ventilation
Transfer to intermediate care unit or intensive care unit
Extracorporeal liver support
Rescue hepatectomy/liver transplantation
Hepatic function
Adequate coagulation (INR < 1.5)
Inadequate coagulation (INR ≥ 1.5, < 2.0)
Inadequate coagulation (INR ≥ 2.0)
No neurological symptoms
Beginning of neurologic symptoms (i.e., somnolence, confusion)
Severe neurologic symptoms/hepatic encephalopathy
Renal function
Adequate urine output (≥ 0.5 mL/kg/h), BUN < 150 mg/dL, no symptoms of uremia
Inadequate urine output (≤ 0.5 mL/kg/h), BUN < 150 mg/dL, no symptoms of uremia
Renal dysfunction not manageable with diuretics, BUN ≥ 150 mg/dL, symptoms of uremia
Pulmonary function
Arterial oxygen saturation > 90 %. May have oxygen supply via nasal cannula or oxygen mask
Arterial oxygen saturation < 90 % despite oxygen supply via nasal cannula or oxygen mask
Severe refractory hypoxemia (arterial oxygen saturation ≤ 85 % with high fraction of inspired oxygen
Additional evaluation
Not required
Abdominal ultrasonography/CT, chest radiography, sputum, blood, urine culture, brain CT
Abdominal ultrasonography/CT, chest radiography, sputum, blood, urine culture, brain CT, intracranial pressure monitoring device
In addition, PHI should sensitively predict postoperative mortality from liver failure. Therefore, the definition of PHI should not include clinical outcomes or ongoing treatment. Among the various definitions of PHI reported in the previous studies, the so-called 50–50 criteria [2] (prothrombin time < 50 % and serum bilirubin level > 50 μmol/L on postoperative day 5) and our definition of PHI [3] (peak postoperative total bilirubin level > 7 mg/dL) are simple and promising objective criteria based on studies including large numbers of patients.
In a multiinstitutional study of 1059 patients without cirrhosis, receiver operating characteristics curve analyses revealed that a peak total bilirubin level of greater than 7 mg/dL was the most sensitive predictor of death from liver failure, with an area under the curve of 0.982 (95 % CI, 0.964–0.999) and a cutoff value of 7.0 mg/dL (sensitivity, 93.3 %; specificity, 94.3 %; accuracy, 94.3 %) (Table 17.2) [3]. In this study, peak total bilirubin level predicted postoperative morbidity (both any morbidity and major morbidity), liver-related mortality, and death from any cause, independent of transfusion status.
Table 17.2
Diagnostic characteristics of various criteria for predicting liver failure-related death. (Reprinted with permission [3] © Elsevier 2007)
Characteristic | Postoperative peak serum bilirubin level > 7.0 mg/dL | Postoperative peak INR > 2.0 | Postoperative peak serum bilirubin level > 7.0 mg/dL and postoperative peak INR > 2.0 | Prothrombin time < 50 % and serum bilirubin level > 50 μmol/L on postoperative day 5 (“50–50 criteria”) |
|---|---|---|---|---|
Sensitivity, n (%) | 28/30 (93.3) | 23/30 (76.7) | 22/30 (73.3) | 14/28 (50.0) |
Specificity, n (%) | 963/1021 (94.3) | 828/1010 (82.0) | 982/1005 (97.7) | 964/997 (96.6) |
Positive predictive value ( n) | 0.326 (28/86) | 0.112 (23/205) | 0.489 (22/45) | 0.292 (14/48) |
Negative predictive value ( n) | 0.998 (963/965) | 0.992 (828/835) | 0.992 (982/990) | 0.986 (964/978) |
Positive likelihood ratio | 17.2 | 4.34 | 32.6 | 15.3 |
Negative likelihood ratio | 0.07 | 0.28 | 0.27 | 0.498 |
Risk Factors for PHI
Reported risk factors for PHI or liver failure are summarized in Table 17.3.
Table 17.3
Risk factors for postoperative hepatic insufficiency. (Reprinted with permission from [31] © John Wiley and Sons)
Surgery related |
Small future liver remnant volume |
Excessive intraoperative blood loss |
Prolonged operating time |
Patient related |
Preexisting liver disease |
Cirrhosis |
Steatosis |
Cholestasis |
Chemotherapy-associated liver damage |
Male gender |
Advanced age (65 years or older) |
Comorbid conditions |
Malnutrition |
Others |
Hepatic parenchymal congestion |
Ischemia–reperfusion injury |
Infection |
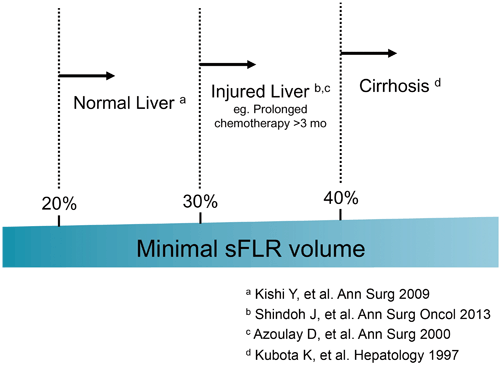
Among the surgery-related factors, small FLR volume is the most important and modifiable factor for patients undergoing extended resection. A strong correlation between small FLR volume and increased risk of PHI is widely recognized, and various FLR volume criteria have been used to select patients who are at high risk of PHI. The poorer the quality of the underlying hepatic parenchyma, the larger the FLR required; therefore, the minimum FLR volume required should be determined according to the status of the underlying liver.
At The University of Texas MD Anderson Cancer Center, we calculate the estimated total liver volume (TLV) using a formula that relies on the linear correlation between the TLV and body surface area (BSA): TLV (cm3) = − 794.41 + 1267.28 × BSA (m2) [4]. The standardized FLR (sFLR) is then calculated as the ratio of the FLR volume to the estimated TLV. In a large cohort study seeking optimal cutoff values for minimum sFLR required, it was estimated that for patients with normal underlying liver, sFLR of at least 20 % is needed to avoid PHI or death from liver failure [5], while for patients who received extensive chemotherapy (≥ 3 months) before surgery, sFLR should be at least 30 % [6]. For patients with liver cirrhosis, it was reported that sFLR should be 40 % or more [7]. Current clinical evidence regarding the sFLR required is summarized in Fig. 17.1.
Another risk factor for PHI is chemotherapy-associated liver damage. Currently, the most common indication for hepatectomy is colorectal liver metastases. Because of advances in effective chemotherapy, the vast majority of patients with colorectal liver metastases are treated with perioperative systemic therapy in combination with surgery. Specific associations between chemotherapy regimens and types of liver injury have been reported. Sinusoidal injury has been associated with oxaliplatin [8, 9], and steatohepatitis has been linked to irinotecan, particularly in patients with high body mass index [10]. In particular, steatohepatitis after major hepatectomy has been correlated with high mortality rates [10]. Chemotherapy-associated liver injuries cannot be accurately predicted, but two factors are known to correlate with increased likelihood of chemotherapy-associated complications: longer duration of preoperative chemotherapy and shorter time interval between the cessation of chemotherapy and surgery. In patients who received chemotherapy for more than 3 months [6], the possibility of hepatic injury should be entertained, and in-depth histopathologic review of the nontumorous liver, volumetry, and laparoscopy should all be considered.
Prevention of PHI
Systematic Volumetry of the “Fully Functioning” Part of the Liver
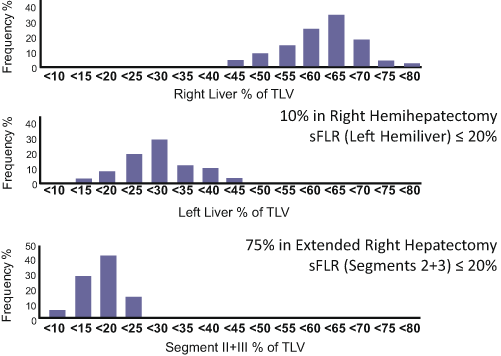
Volumetry of the liver is essential to assess the risk of PHI. A previous anatomic study revealed that the left lateral bisegments account for only 16 % of the total liver volume (Fig. 17.2) [11]. Thus, routine volumetry using an adequate method is recommended, especially in patients undergoing extended right hepatectomy. FLR volume should be defined as the absolute volume of the “fully functioning” part of the liver, in other words, the part of the liver that will have adequate inflow and outflow after hepatectomy. When a hepatic vein draining a specific part of the liver is deprived, the corresponding part of the liver will be congested and will atrophy because of loss of its normal function [12, 13]. A recent study using indocyanine fluorescent technique revealed that portal uptake function in the venoocclusive part of the liver is approximately 40 % of that in the nonocclusive part of the liver [14]. However, precise estimation of the volume of the area to be congested is difficult without the use of three-dimensional liver simulation techniques (Fig. 17.3) [15, 16]. Therefore, on volumetry for patients undergoing extended right hepatectomy in which the middle hepatic vein will be deprived, segment IV should not be included in the FLR volume because most of segment IV will be congested and lose its normal function after deprivation of the middle hepatic vein even when part of segment IV is preserved.
 < div class='tao-gold-member'>
< div class='tao-gold-member'>





Only gold members can continue reading. Log In or Register to continue
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree