Chapter 1.3
Physiology and function of the stomach
Luca Marciani and Mark Fox
University of Nottingham, Nottingham, UK
1.3.1 Physiology, anatomy and function
The human stomach is a J-shaped organ of the gastrointestinal (GI) tract, located between the oesophagus and the duodenum, and it has a key role in digestion and absorption. The main anatomical regions are shown in Figure 1.3.1. The stomach’s main functions are to store and break down food and deliver digesta to the small intestine.
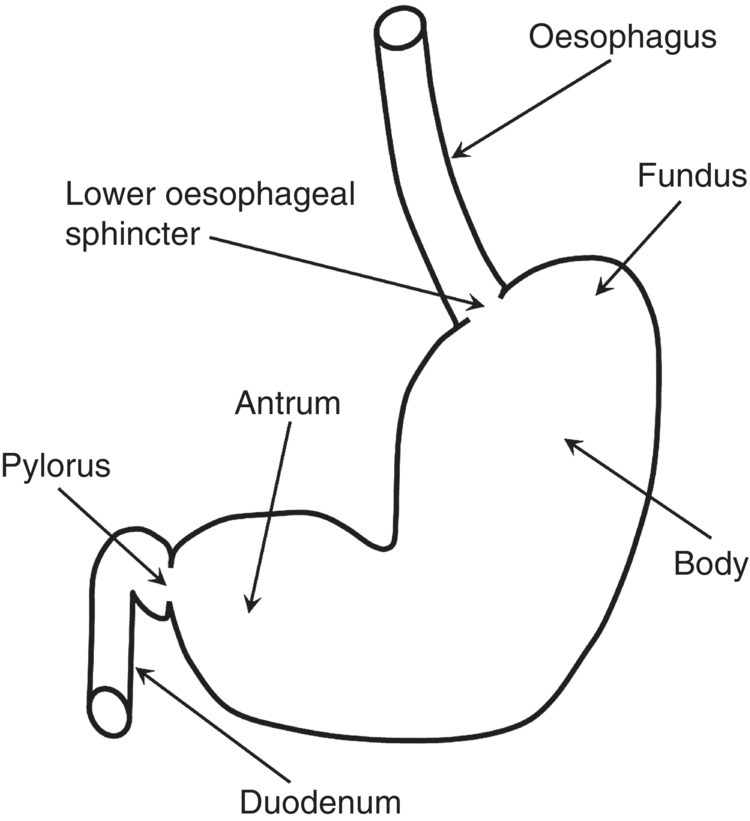
Figure 1.3.1 Schematic diagram of the human stomach.
The stomach receives boluses of food via the lower oesophageal sphincter. It is able to reduce gastric wall tone via a vagally mediated reflex (‘accommodation’) which allows the reservoir to expand and accommodate increasing amounts of food without important increases in intragastric pressure [1]. In addition to ‘receptive’ accommodation mediated by mechanoreceptors in the gastric wall, once nutrients pass into the small intestine the gastric response is modulated by chemoreceptors and osmoreceptors to ensure that gastric emptying through the pylorus is controlled and optimized for efficient digestion [1,2].
During intragastric food processing, the stomach secretes hydrochloric acid, lipase and pepsin. This process is regulated by the central and enteric nervous system and neuroendocrine cell networks [3]. These secretions together with salivary enzymes active within the bolus start the chemical breakdown of food. At the same time, highly co-ordinated antropyloroduodenal contractions effect mechanical breakdown (trituration) of solid food. Gastric emptying is ultimately the result of these co-ordinated actions, controlled opening of the pylorus and antroduodenal differences in pressure which drive gastric emptying [4,5]. Liquids empty faster than solids, which are first triturated to small particles, usually less than 3 mm in size, to promote chemical digestion and absorption after delivery to the duodenum and small intestine [6]. Other physical factors such as meal viscosity, the density and breaking strength of food particles also affect the rate of gastric emptying [6–8].
1.3.2 Measurement and assessment of gastric function
Measurement of gastric function has improved understanding of the physiological response to food in health and disease and in response to dietary or pharmacological intervention. A number of tests are available and are briefly described in the following sections [9].
Gastric accommodation and sensation
Gastric accommodation can be evaluated using the barostat test. This involves intubating the subjects orally using a double-lumen catheter with a plastic bag on the tip. The balloon is commonly placed in the proximal stomach. An electronic barostat device is then used to control expansions of the bag to assess, for example, volume expansion during pressure-guided distension or after delivery of a test meal [10]. This is the ‘standard test’ of gastric accommodation though availability is limited, the method is invasive and the presence of a balloon in the stomach affects gastric relaxation. Gastric sensation elicited by barostat distension paradigms leads to brain cortical activations that can be assessed using functional brain magnetic resonance imaging (MRI) and positron emission tomography (PET) methods [11,12].
A simple and inexpensive alternative to the barostat is the drink test [13]. This involves ingesting water or a nutrient drink at a given rate until the maximum tolerated volume is reached. Subjective scores of sensation are collected during and after the test. The results are not easy to interpret due to variation in gastric capacity and the merits of this test are debated.
Conventional ultrasound has been used to measure the area of the proximal stomach after a meal in a sagittal section and the maximal diameter in an oblique frontal section [14]. Three-dimensional reconstruction of ultrasound images integrates this information and gives volume measurements; however, the technique is user dependent and can be used only with liquid meals.
The distribution of gastric contents within the stomach on scintigraphy provides some impression of gastric accommodation [15]. Another nuclear medicine test that can measure change in gastric volumes is single photon emission computed tomography (SPECT). This method involves injecting intravenously a 99mTc-labelled compound which is taken up in the mucosa. A dual-headed gamma camera is used to measure the radiation emitted and reconstruct axial images of the stomach. A three-dimensional image can be reconstructed later; however, the temporal and spatial resolution are limited compared to MRI.
Magnetic resonance imaging is an emerging technique used to assess fasting and postprandial gastric volumes [16] due to the lack of ionising radiation, multiplanar imaging, speed and excellent contrast between different organs and intragastric meal components. It has been used to evaluate the effects of the barostat balloon in the stomach [17], finding that the bag increased postprandial gastric volumes. Cross-sections of the fundus [18] and maximum antral diameters following model meals [7] have also been measured using MRI and changes in these variables correlate closely with sensation of fullness and other symptoms in health and disease [8,19].
Gastric contractility
Antroduodenal motility can be measured using intraluminal manometry by passing a catheter nasogastrically through the pylorus and into the proximal duodenum. The catheter has a varying number of water-perfused or solid-state sensors. These detect the periodical stomach wall contractions and the pressure amplitude profiles with time can be displayed and analysed [20].
The high-resolution and high-speed capabilities of MRI allow imaging of the stomach serially at intervals of a few seconds. These images can be played as motility ‘movies’ and subsequently postprocessed to measure motility in terms of antral contractions, frequency, speed and percentage occlusion [21–24]. An interesting finding from MRI studies is the lack of correlation between meal volumes and antral contractility that suggests these contractions are highly stereotyped after a meal and do not determine the rate of gastric emptying through the pylorus [5,25]. Dynamic gamma scintigraphy can measure antral motility but this requires higher radiation doses and the resolution is poor.
Gastric emptying
Gastric emptying can be measured by labelling test meals with 13C stable isotopes such as octanoic acid. The label is absorbed in the small intestine during digestion, metabolised to 13CO2 and then expelled with the breath. As such, serial breath samples are taken at baseline and postprandially to calculate the increase of 13CO2 with time, which is then assumed to be proportional to gastric emptying [26]. This is an advance on the oral paracetamol absorption under the assumption that the appearance in the blood is directly related to gastric emptying [27].
Using imaging, the simple radiopaque marker test involves the subject ingesting a number (about 20) of small radiopaque pellets with a test meal and following their emptying with fluoroscopy [28]. Results depend on the size and density of the pellets and test meal composition.
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree





