Chapter 3.5
Gastric cancer and nutrition
Clare Shaw
Royal Marsden NHS Foundation Trust, London, UK
3.5.1 Incidence and aetiology
An estimated 990,000 people were diagnosed with gastric cancer worldwide in 2008, accounting for 8% of the total cancer diagnoses [1]. The incidence of gastric cancer varies around the world with the highest rates occurring in eastern Asia and the lowest rates in northern and southern Africa [1]. The incidence of gastric cancer worldwide is more than double in men than in women (Figures 3.5.1, 3.5.2). Rates of gastric cancer have been declining worldwide for several decades which is thought to be related to improvements in diet, food storage and preservation which may be linked to a decrease in the prevalence of Helicobacter pylori.
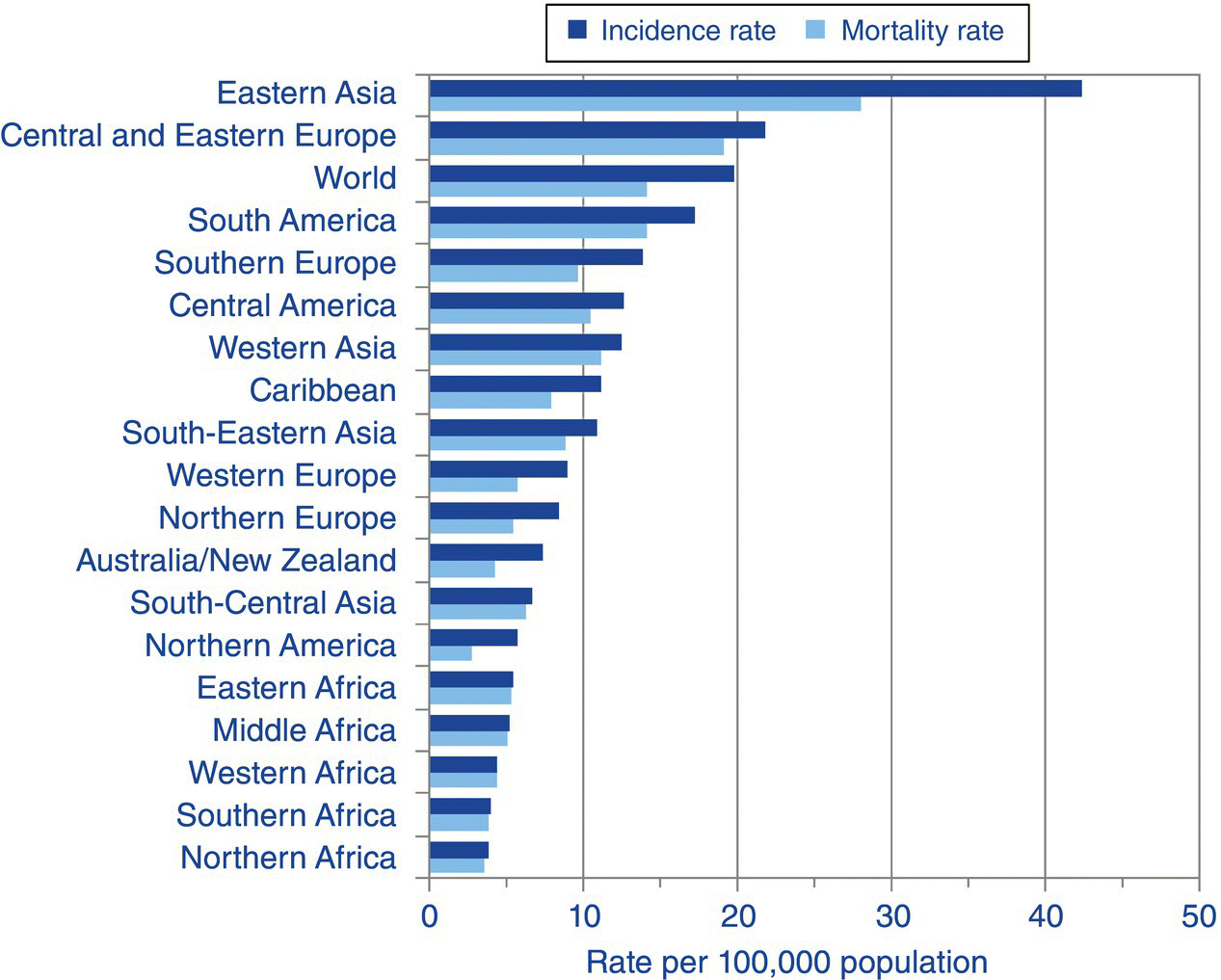
Figure 3.5.1 Stomach cancer: world age-standardised incidence and mortality rates, males, regions of the world, 2008 estimates [1]. Reproduced with permission from Cancer Research UK.
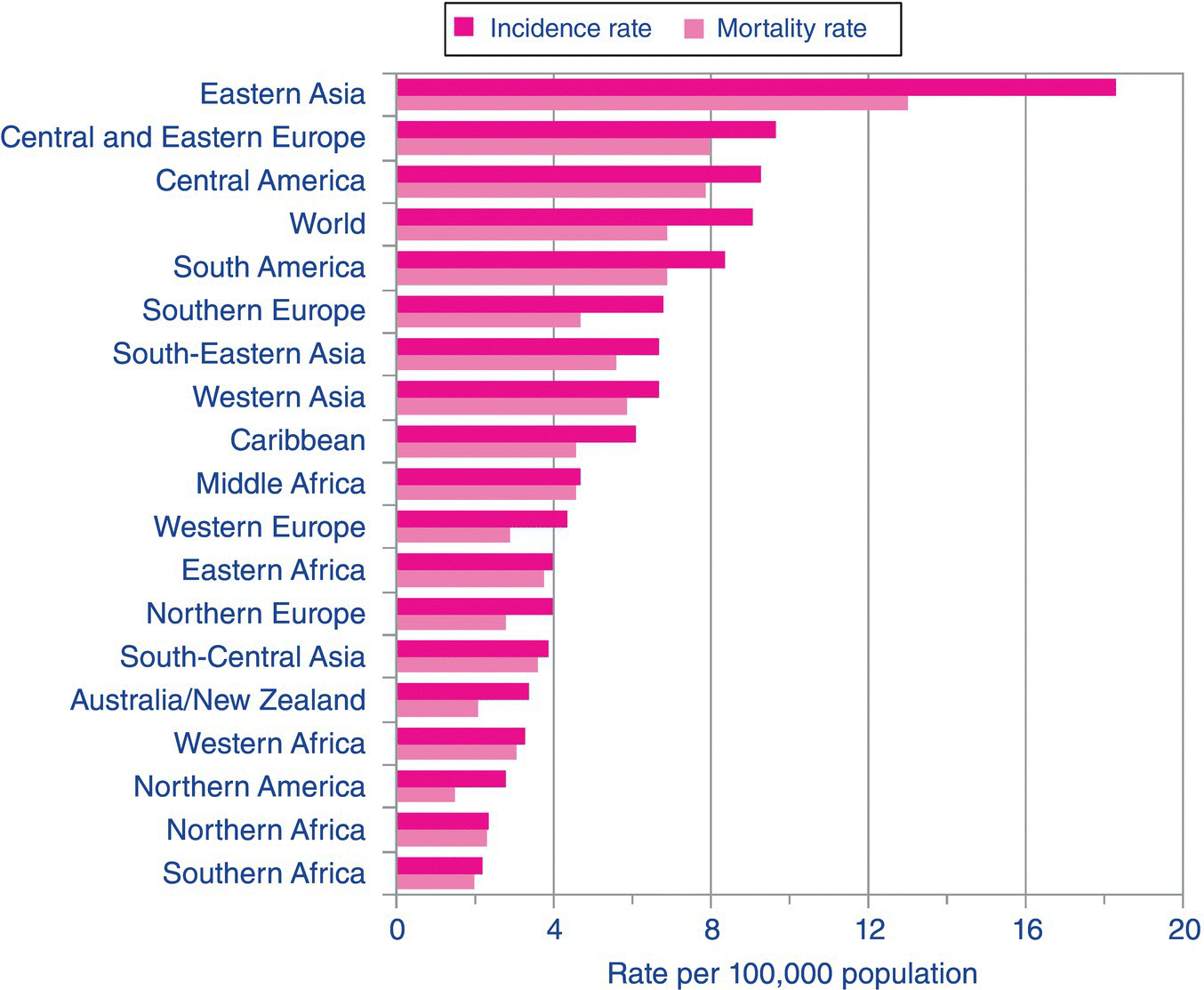
Figure 3.5.2 Stomach cancer: world age-standardised incidence and mortality rates, females, regions of the world, 2008 estimates. Reproduced with permission from Cancer Research UK.
Over half of gastric cancers are caused by the bacterium Helicobacter pylori which was discovered in 1984 by Barry Marshall and Robin Warren [2]. It was initially found to be associated with gastric ulcers and gastritis but has also been identified as a cause of gastric cancer. Particular strains of H. pylori predispose to gastric cancer, possibly through a number of mechanisms including inflammation of gastric epithelium, stimulation of inflammatory cells and cellular changes induced by injection of protein products into the epithelial cells of the stomach [3]. These cellular influences in conjunction with environmental factors, including diet, eventually support the growth of malignant cells [4]. There may be a genetic component to these changes with individuals who readily produce cytokines, particularly interleukin-8, being at increased risk of subsequently developing gastric cancer [5].
Other environmental factors that increase the risk of gastric cancer include smoking, with approximately 20% of gastric cancers being attributed to tobacco smoking [6].
Dietary intake also influences the development of gastric cancer. High consumption of salt, as assessed by salt added to food and consumption of processed meat, increases the risk of development of gastric cancer [7]. Other studies have estimated total consumption of salt using a food frequency questionnaire, estimation of salt intake from main food groups and the use of added salt at the table and these too have found an association between higher salt intakes and an increased risk of gastric cancer [8]. High intakes of vegetables may have a protective effect against gastric cancer [9]. Allium vegetables in particular may have a preventive effect, possibly due to a direct action against H. pylori [10,11]. Case–control investigations in the European Prospective Investigation into Cancer and Nutrition (EPIC) study examined concentrations of carotenoids, retinol and alpha-tocopherol in individuals in prediagnosis blood samples compared to controls not diagnosed with gastric cancer. The results showed that higher plasma concentrations of some carotenoids, retinol and alpha-tocopherol are associated with reduced risk of gastric cancer [12]. It is thought that the protective effect of carotenoids may be due to their antioxidant properties, limiting DNA damage and oxidative stress. Retinol may have an effect on the control of cellular growth [12].
There is interest in the potential of chemoprevention for gastric cancer although, as yet, the ideas are not supported by research evidence [13]. Cyclo-oxygenase-2 (COX-2) is an enzyme responsible for the production of prostaglandins and prostacyclins that are involved in the inflammation cascade, and increased concentrations of COX-2 are present during the progression of atrophic gastritis to intestinal metaplasia and gastric cancer. Environmental factors such as smoking, increased gastric acid production and H. pylori are all associated with greater COX-2 expression. Aspirin and other non-steroidal drugs inhibit COX-2 and therefore have been proposed as possible chemopreventive drugs for gastric cancer. There is interest in whether anti-inflammatory fatty acids are also able to modulate this response and work has been carried out in animal models [14]. The results of clinical trials in humans are awaited.
3.5.2 Diagnosis and staging
The presenting symptoms of gastric cancer can range from mild gastritis or indigestion to gastric outflow obstruction, the latter having a significant impact on nutritional status as it causes vomiting and severely impairs adequate dietary intake. Other symptoms may be non-specific and include nausea, anaemia, loss of appetite, fatigue and weight loss [15,16].
All patients with suspected gastric cancer require an endoscopy and, for staging of the disease, all patients should undergo a computed tomography (CT) scan plus a staging laparoscopy. Gastric cancer is staged using the TNM system to describe the size and spread of the tumour and if it has spread to the submucosa or muscle wall or penetrated the stomach wall (T). The N and M denote whether it has spread to lymph nodes (N) and to other parts of the body (M).
3.5.3 Treatment
Treatment of gastric cancer will depend on the stage of the disease and the performance status of the patient which measures general health and ability to perform activities of daily living. Other factors affecting treatment choice include whether any co-morbidities, such as heart disease, are present. Decisions on the preferred treatment should be made after appropriate staging of the disease and an assessment by members of the multidisciplinary team [13].
Chemotherapy and targeted therapies
Perioperative chemotherapy confers a survival advantage when compared to surgery alone and therefore this is the preferred course of treatment [13]. Some patients may not be suitable for surgery and therefore may benefit from palliative chemotherapy that has been demonstrated to improve health-related quality of life and survival [13]. If cells have human epidermal growth factor receptors (HER2) then the patient may also be treated with trastuzumab, a targeted treatment which has been demonstrated to improve disease-free survival.
Surgery
Surgical resection should only be undertaken in patients who are sufficiently well with a good performance status who are able to withstand a surgical intervention. The resection depends on the site of the tumour and may include a subtotal gastrectomy for distal tumours or a total gastrectomy for proximal tumours. Oesophagogastric junctional tumours require a transhiatal extended total gastrectomy or oeosphagogastrectomy [13]. Additional lymph nodes should be removed, with the extent of this resection depending on the performance status of the patient and the position of the tumour.
Alternatively, surgery may be used with palliative intent to relieve symptoms, such as gastric outflow obstruction, in patients who are not suitable for a curative resection. As with all cancer patients undergoing surgery, nutritional status should be optimised before surgical treatment to reduce morbidity and mortality [17].
Radiotherapy
Radiotherapy may be used in combination with chemotherapy to improve survival and is considered in patients who are at high risk of recurrence and who have not received neoadjuvant therapy, i.e. any form of cancer treatment prior to the main treatment modality. Palliative radiotherapy may be appropriate for some patients depending on their symptoms and performance status. Planning and delivery of such treatment in a palliative setting should always be done in the context of a multidisciplinary team with palliative care support for symptom management.
3.5.4 Nutritional status of gastric cancer patients
Cancer cachexia, a term that describes nutritional and inflammatory changes in the patient, is common in upper GI cancer [16,18]. The relative contributions of changes in dietary intake and inflammation caused by the cancer itself are difficult to ascertain and it is likely that in this group of patients both are contributory factors to changes in body composition.
Weight loss is common at the time of diagnosis of gastric cancer and is influenced by GI symptoms and a reduced dietary intake. In a study of 220 patients with upper GI cancer, 83% had lost weight at the time of diagnosis which amounted to a median loss of 7% of body weight [19]. This equated to a mean weight loss of 2.5% per month prior to diagnosis. In this group of patients 39% had lost more than 10% of their premorbid body weight. In this study, weight loss was associated with advanced disease, difficulty eating and poor dietary intake.
Gastric cancer can have a profound effect on dietary intake that may continue during and after treatment [19]. Symptoms often present in gastric cancer include abdominal pain, anorexia, dysphagia, nausea and vomiting [20]. Gastric outflow obstruction may occur if the tumour is situated near the pylorus. This results in gastric distension, satiety, nausea and vomiting with an inadequate dietary intake.
The metabolic changes of cancer cachexia may influence both protein and fat metabolism. There may be both host and tumour factors that reduce protein synthesis and increase protein degradation, resulting in a preferential loss of skeletal muscle mass [21]. Fat stores in the body are mobilised, possibly as a result of negative energy balance but also due to the action of intermediary metabolites such as lipid-mobilising factor (LMF) or tumour necrosis factor (TNF) alpha.
In gastro-oesophageal cancer patients, high serum C-reactive protein, a measure of inflammation, is also associated with weight loss which indicates that changes in dietary intake and the inflammatory response are both present in patients [19]. Lack of studies specifically measuring metabolic rate in gastric cancer patients prior to treatment makes it difficult to ascertain the relative contribution, if any, of these metabolic changes on energy expenditure and potentially weight loss.
The method of nutritional support is an important consideration for patients in their treatment pathway. If patients are palliative then a pyloric or duodenal stent may be appropriate to manage an obstruction. If patients are being treated with curative intent then they may require nutritional support whilst undergoing chemotherapy, radiotherapy or being prepared for surgery. These decisions should be taken following a full staging of the cancer and a multiprofessional discussion on the treatment plan and support required [13].
Nutritional and performance status can influence treatment options. When both are poor, patients are less able to withstand treatment side-effects. Weight loss is associated with poor tolerance to chemotherapy with increased side-effects, longer breaks during treatment to allow the patient to recover and overall a reduction in the quantity of chemotherapy given to patients [22,23]. Lean body mass is important for the distribution of cytotoxic drugs so increased toxicity to chemotherapy may occur as a result of lower fat-free mass. In a study of patients with lung or GI cancer undergoing 5-FU chemotherapy, those with sarcopenic obesity, and loss of lean body mass, had increased toxicity and poorer survival following chemotherapy [24].
3.5.5 Nutritional support
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree





