Criteria
Grade A fistula
Grade B fistula
Grade C fistula
Clinical conditions
Well
Often well
Ill-appearing/bad
Specific treatment
No
Yes/no
Yes
Ultrasound/CT scan
Negative
Negative/positive
Positive
Persistent drainage (> 3 weeks)
No
Usually yes
Yes
Signs of infection
No
Yes
Yes
Sepsis
No
No
Yes
Reoperation
No
No
Yes
Readmission
No
Yes/no
Yes/no
Death related to fistula
No
No
Yes
With this three-category system, a standardized definition was established, which was widely accepted, validated, and used worldwide by all major study groups for the categorization of patient data. Pratt et al. prospectively analyzed postoperative complications in 176 patients after pancreaticoduodenectomy [10]. In this study, there were 53/176 patients (30 %) confirmed fistula—26 (15 %) type A, 21 (12 %) type B, and 6 (3 %) type C. Patients with grade A fistula had shorter hospital stays and less secondary complications than patients with grade B and C fistula. Compared to patients with grade B fistula, patients with grade C fistula had a longer hospital stay, a higher frequency of intensive care unit (ICU) admissions, and more blood transfusions. This study served to validate the ISGPF classification scheme in demonstrating minimal clinical impact of type A fistulas, while showing more complications and costs in patients with type B and C fistula.
Procedure-Specific Incidence and Risk Factors for Pancreatic Fistula
The occurrence of a pancreatic fistula is highly dependent on the type of surgical procedure performed and the underlying pancreatic pathology. Soft pancreatic tissue texture without pre-existing fibrosis is regarded as a risk factor for fistula development in all pancreatic procedures.
Pancreaticoduodenectomy
Pancreaticoduodenectomy is the treatment of choice for patients with resectable carcinoma of the pancreatic head and periampullary region. In recent years, the mortality rate of pancreaticoduodenectomy has declined to < 5 %. However, the overall morbidity remains at approximately 50 % with the pancreatic fistula occurring in 5–40 % of patients [6, 7]. In an attempt to understand pancreatic fistula after pancreaticoduodenectomy, several risk factors have been identified. These include patient risk factors (age, sex, bilirubin level, and comorbid conditions), pancreas risk factors (pancreatic texture, pancreatic duct size, underlying patient pathology and blood supply to the pancreatic remnant) and operative risk factors (operative time, blood loss, anastomotic techniques, and stent usage). Evaluation of these risk factors led to the generally accepted theory that a fibrotic pancreatic remnant facilitates the pancreaticoenteric anastomosis, whereas, a soft pancreatic remnant frequently results in a higher pancreatic fistula rate.
Recently, a single 10-point fistula risk score (FRS) was developed, for the prediction of critically relevant postoperative pancreatic fistula (CR-POPF) after pancreaticoduodenectomy using risk factors from the ISGPF classification [11]. Based on an extensive analysis of pre- and intra-operative variables, four distinct factors were discovered: pancreatic duct size smaller than 3 mm; soft pancreatic parenchyma; ampullary, duodenal, cystic, or islet cell pathology; and excessive intraoperative blood loss (Table 28.2). An aggregate of 0–10 points subsequently determines a patient’s fistula risk profile. Patients with 0 points have a negligible risk to develop a biochemical fistula or CR-POPF. Patients with 1–2 points have low-risk (14 %) of developing any fistula with less than one-third developing CR-POPF. Patients who accumulate between 3 and 6 points are in intermediate risk and 25 % can be expected to develop pancreatic fistulas, which are twice as likely to be clinically relevant. Finally, patients who acquire 7 or more points are considered high risk, because the incidence of CR-POPF approaches 90 %. This FRS has been internally and externally validated by a multi-institutional study that confirmed that the FRS was a strong prognostic tool for predicting the development of CR-POPF after pancreaticoduodenectomy [12].
Table 28.2
Fistula risk score for prediction of clinically relevant pancreatic fistula after pancreaticoduodenectomy. (Adapted from [11])
Risk factor | Parameter | Pointsa |
|---|---|---|
Pathology | Pancreatic adenocarcinomaor pancreatitis | 0 |
Ampullary, duodenal,cystic, islet cell | 1 | |
Gland texture | Firm | 0 |
Soft | 2 | |
Intraoperative blood loss, mL | ≤ 400 | 0 |
401–700 | 1 | |
701–1000 | 2 | |
> 1000 | 3 | |
Pancreatic duct diameter, mm | ≥ 5 | 0 |
4 | 1 | |
3 | 2 | |
2 | 3 | |
≤ 1 | 4 |
Distal Pancretectomy
Distal pancreatectomy is performed for all kinds of pancreatic pathologies, including chronic inflammation and benign and malignant tumors. Pancreatic fistulas are merely leakage of pancreatic fluid from the cut margin of the pancreatic remnant. The average reported pancreatic fistula rates following distal pancreatectomy are approximately 20–25 % ranging from 0 to 40 % with approximately 97 % of these being type A or type B fistulas [13, 14, 15]. Many different factors like surgical stump management, spleen preservation, tissue texture, or extent of surgical procedure can have an impact on fistula development and have been investigated in numerous studies. The technique of stump closure after distal pancreatectomy remains the subject of an ongoing debate. All approaches including fibrin glue, sealants, patches, stapler closure, electrocautery, and suture have been tested in numerous studies. In an analysis by Ferrone et al. of 462 patients, fistula rates were 19–31 % considering all approaches without significant advantages for any method [14]. The recently completed DISPACT trial included 352 patients that were randomly assigned to a stapler or hand-sewn closure of the pancreatic remnant. Both groups showed identical fistula rates of 30 and 36 % on postoperative day 7 and 30, respectively [15].
The role of splenic preservation on fistula development is also controversial. An analysis of 211 patients by Shoup et al. showed that splenectomy was associated with a higher risk for clinically-relevant fistula [16]. In contrast to this publication, the two large series by Kleeff et al. including 302 patients and by Lillemoe et al. with 235 patients, failed to confirm splenectomy as an independent risk factor for fistula development [17, 18].
In recent years, laparoscopic distal pancreatectomy for benign as well as malignant disorders has gained acceptance. In a multicenter study of 96 laparoscopic distal pancreatectomies, the overall fistula rate was 17 % [19]. In contrast, a meta-analysis published in 2009 which included 28 studies, found an overall fistula rate of 29 % [20]. When compared to the open approaches in a multicenter study of 142 laparoscopic versus 200 open resections, a rather high fistula rate (26 % laparoscopic vs. 32 % open) was reported [21]. Currently, there is no evidence supporting the laparoscopic procedure over the open procedure with regard to postoperative fistula development.
Duodenum-Preserving Pancreatic Head Resection/Lateral Pancreaticojejunostomy
Duodenum-preserving pancreatic head resection (DPPHR) and lateral pancreaticojejunostomy (Puestow procedure) are the procedures used in the surgical treatment of chronic pancreatitis. These patients usually show a fibrotic tissue texture which facilitates surgical tissue handling and is associated with a reduced risk for anastomotic leak. Although all of these procedures require anastomotic suture line of extensive length, the fistula rates are low ranging from 0 to 6 % [22]. There are no clear advantages with regard to fistula development for any of the common DPPHR modifications (Frey or Beger) or the Puestow procedure which supports the fact that fistula development is mainly dependent on the fibrotic pancreatic texture, not on the surgical technique or the extent of resected tissue in these procedures.
Pancreatic Pseudocyst Drainage/Pancreatic Necrosectomy
Acute necrotizing pancreatitis is the most severe and potentially life-threatening form of acute pancreatitis. As many cases involve infection of the necrotic tissue, almost all will require percutaneous drainage of peripancreatic fluid collections or a surgical procedure for the debridement of necrosis (necrosectomy) (Fig. 28.1a, b). In acute necrotizing pancreatitis, the pancreatic duct often disrupted by the necrosis, results in creation of a pseudocyst or either a sterile or an infected collection consisting of pancreatic juice and necrotic tissue. Any procedures to address these fluid collections by external drainage will result in an external pancreatic fistula.
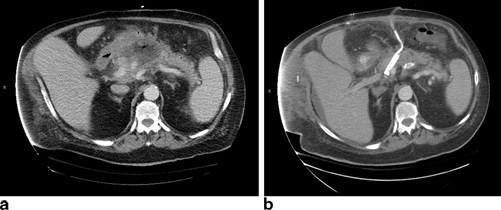

Fig. 28.1
a Patient with infected pancreatic necrosis. b Same patient after operative debridement and drainage of pancreatic necrosis
Howard et al. have classified external pancreatic fistulas anatomically into end and side fistulas. Side fistulas can be further classified as postoperative and inflammatory [23]. End external pancreatic fistulas are leaks from the pancreatic duct which have no continuity with the gastrointestinal tract. The most common anatomic configuration in these is the “disconnected duct syndrome” due to necrosis of the midpancreatic body along with the ductal epithelium, with no communication between the external pancreatic fistula and the proximal pancreatic duct. The distal remnant of the pancreas is an isolated pancreatic segment draining only via the fistula. All such end fistulas will require either internal drainage or resection in order to close.
A pancreatic fistula following percutaneous drainage of a pseudocyst occurs approximately 15 % of the time [23]. Persistent drainage is often the result of an obstructing stricture within the main pancreatic duct, causing the pressure within the duct to be abnormally high. Patients with pancreatic necrosis secondary to acute pancreatitis often present with pancreatic duct disruption. At the time of the initial surgery, the goal is to debride all necrotic tissue and perform wide drainage. Most duct disruptions go on to seal with time and drainage, however 10–56 % continue to have persistent drainage that may require more definitive management [24].
Other Pancreatic Resections
Middle segmental pancreatic resections are tissue-sparing procedures usually employed for benign pancreatic neoplasms. Current literature reporting results from nearly 300 patients, reports fistula rates between 10 and 40 % with most series having rates higher than 25 % [25, 26]. This high rate is explained by the existence of two cut pancreatic surfaces, which are either closed by an anastomosis or by duct/parenchyma closure comparable to distal resections.
Tumor enucleations of the pancreas represent another type of resection with a rather high reported fistula incidence. In a 61-patient study by Crippa et al. fistula incidence was 23 %, while smaller series report fistula rates of approximately 40 % [27, 28]. Despite these rather high overall fistula rates, associated complications are low in all studies. Grade C fistulas range between 0 and 4 %, showing that enucleation-associated fistula are rare and treated by drainage without further specific therapy.
The pancreatic fistula after operative trauma is usually isolated to the tail of the pancreas following splenectomy, left nephrectomy/adrenalectomy, and mobilization of the splenic flexure during colectomies. Reported fistula rates range from 0 to 2 % after these operations [27].
Prevention of Pancreatic Fistula
Given the frequency of pancreatic fistulas following pancreatic resection, extensive research has been employed to prevent the occurrence of pancreatic fistula. The strategies include pharmacologic manipulation, modifications and refinements in surgical technique regarding pancreatic anastomosis, pancreatic anastomotic stents, and perianastomotic drainage post pancreatic resection.
Octreotide, a synthetic somatostatin analogue inhibits pancreatic exocrine secretion. The use of octreotide and its analogues to prevent postoperative fistula is an approach which has been used since the 1990s [29, 30]. Despite 20 years of clinical use and evaluation in numerous studies, a recent Cochrane meta-analysis concluded that evidence is still lacking to give clear guidelines [31]. While early randomized controlled trials (RCTs) favored the use of octreotide and showed a 50 % reduction of fistula rates, these findings were not confirmed in later studies [30, 32, 33]. From these results, it was concluded and supported by the Cochrane review that routine use of octreotide was not indicated, but should be used in a risk-dependent manner in a presumed “critical” anastomoses due to soft pancreatic tissue texture. Although the overall fistula rates have been reduced, somatostatin analogues failed to reduce the incidence of clinically relevant (grade B/C) fistula or re-operation rates and mortality. Finally, postoperative octreotide administration for postoperative fistula has failed to show any improvement in the rate of fistula closure [34]. Despite the lack of effect on fistula closure rate, the octreotide may help lower fistula output and make fistula control easier. Recently, a single-center, randomized, double-blind trial was published showing pasireotide, a new somatostatin analogue, decreased the rate of clinically significant postoperative pancreatic fistula, leak, or abscess [35]. While the initial results are promising, further studies need to be conducted to prove whether pasireotide is beneficial.
Modifications in surgical technique to prevent pancreatic fistula have been evaluated for decades with conflicting results. Following a pancreaticoduodenectomy, there has been an ongoing debate of whether a pancreaticogastrostomy or pancreaticojejunostomy has a lower postoperative fistula rate. Within each of these techniques, several different technical modifications, including single or double layer sutures, invagination and purse-string sutures have been compared with regard to the surgical complications and especially postoperative fistula frequency. Unfortunately, there have been no level 1 evidence-supported techniques that have been universally adopted. Following distal pancreatectomy, there are numerous studies comparing sutures, staplers, patches, fibrin glue, and sealants to handle the distal stump of the pancreatic remnant that have been reported [13, 14], and no convincing evidence exists to support the superiority of any one technique.
The placement of pancreatic duct stents and the potential role in prevention of postoperative pancreatic fistula has been investigated for both right and left pancreatic resections. The principle of internal drainage of the pancreatic duct following pancreaticoduodenectomy to achieve a diversion of the pancreatic secretion from the suture site has been hypothesized; however, the stent may also cause problems via irritation of the duct and the suture lines as well as the obstruction or migration. In available studies, the outcome shows a great deal of variability. Earlier studies demonstrated a beneficial effect of anastomotic stenting in lowering the postoperative pancreatic fistula rate [36, 37]. A randomized trial by Poon et al. among 120 patients, which used long stents across the pancreaticjejunostomy anastomosis and drained externally, showed that the stented group had a significantly lower pancreatic fistula rate compared to the nonstented group (6.7 vs. 20 %, respectively) [37]. Despite these encouraging results, the technique has not been universally adopted. In contrast, the largest randomized study, published in 2006 by Winter et al., which used short internalized stents (6 cm long plastic pediatric feeding tube) included 234 patients who underwent pancreaticoduodenectomy with stent ( n = 115) or without stent ( n = 119) placement into the pancreatic duct [38]. Winter showed an overall fistula rate of 7.6 % (no stent) vs. 11.3 % (stent), concluding no benefit for stenting of the pancreatic duct. The most recent study, published in 2012 by Sachs et al. where 59/444 patients had an intraoperatively pancreaticojejunal stent placed actually had greater rates of critically relevant postoperative pancreatic fistula, major complications, greater length of stay, and total costs [39].
There are very few studies analyzing the effect of preoperative or intraoperative stents in the distal pancreatectomy setting. In a 23-patient collective, Fischer et al. described a prophylactic intraoperative transampullary stent placement as an open surgical procedure that resulted in a significant reduction in postoperative pancreatic fistula rates [40]. However, Okamoto et al. observed a stent-related morbidity of 57 %, including pancreatitis and stent obstruction [41]. Reider et al. had no postoperative pancreatic fistula in stented patients; however, in this study a sphincterotomy was performed in addition to the placement of preoperative stents prior to distal pancreatectomy [42].
The routine use of intraperitoneal drains following elective pancreatic surgery remains an area of debate regarding whether drains prevent or exacerbate pancreatic fistulas. The first randomized trial to investigate the impact of intraperitoneal drain use reported by Conlon et al. randomized 179 patients following pancreaticoduodenectomy and distal pancreatectomy to intraperitoneal drain placement or surgery without drains [43]. This study demonstrated that patients in the drainage group were more likely to have an intra-abdominal abscess, collection, or fistula compared to patients without drains. More recently, Bassi et al. randomized patients who were at low risk for leak based on drain amylase level < 5000 U/L on postoperative day 1 to early drain removal (postoperative day 3) or late drain removal (≥ postoperative day 5) [44]. The trial reported a significant reduction in pancreatic fistulas (using the ISGPF definition) in early drain removal group versus the late drain removal group 1.8–26.3 %. While these studies show that drains may not be necessary, most surgeons routinely still use postoperative drains and have not changed their clinical practice. Recently, a randomized multicenter trial was conducted to further evaluate the necessity of drains after pancreaticoduodenectomy [45]. There were no differences between the drain and no-drain cohorts in demographics, comorbidities, pathology, pancreatic duct size, pancreas texture, or operative technique. Pancreaticoduodenectomy without intraperitoneal drainage was associated with an increase in the number of patients with complications, number of complications per patient, and the severity of complications. The no-drain cohort also had a higher incidence of gastroparesis, intra-abdominal fluid collection, intra-abdominal abscess, and severe diarrhea. Furthermore, patients in the no-drain group more often required postoperative percutaneous drains and had a prolonged hospital stay. The Data Safety Monitoring Board stopped the study early because of the increased mortality from 3 to 12 % in the patients undergoing pancreaticoduodenectomy without intraperitoneal drainage. Thus, most surgeons feel that this level 1 evidence provides strong support for routine drainage following pancreaticoduodenectomy. This same study continues to determine the necessity of drains for patients after distal pancreatectomy.
Complications of Pancreatic Fistula
Multiple studies have demonstrated that patients with pancreatic leak have a significant increase in secondary complications compared to patients without leak [8, 46]. Commonly observed complications are mainly caused by undrained infected pancreatic fluid collections. Pancreatic fluid is an enzymatically active and aggressive substance that may cause erosion of the surrounding tissue, organs, and blood vessels. This can lead into leakage from other adjacent anastomoses or bowel (particularly in patients with pancreatic necrosis) causing a biliary, gastric, or enteric leak.
Postoperative hemorrhage associated with a pancreatic leak is one for the most dreaded complications following major pancreatic resections. The pancreatic enzymes in combination with infection can cause erosion of the gastroduodenal artery or splenic artery stump or from an arterial pseudoaneurysm resulting in significant bleeding requiring immediate therapy. This complication usually occurs after the first week after the surgery and in most cases with what appears to be adequate drainage of the pancreatic leak. Management is guided by the patient’s clinical status and hemodynamic stability. In general, most patients should be approached via angiographic embolization or arterial stenting to provide the best outcomes [47]. Patients who are hemodynamically unstable may require operative re-exploration and packing and then angiographic control. A high index of suspicion should be maintained because postoperative hemorrhage is associated with significant risk.
The occurrence of pancreatic fluid collections due to a pancreatic leak is also a potential cause of ongoing abdominal sepsis that can lead to generalized systematic organ failure. Percutaneous drainage of the fluid collections by interventional radiology and broad spectrum antibiotic therapy are as important as supportive ICU therapy in these patients.
An important aspect of the pancreatic fistula complications is the economic impact from the prolonged treatment. The longer duration of hospital stay is an important factor that increases treatment costs. The average hospital stay in uncomplicated resections is usually 6–8 days, but can increase to 25–40 days in cases of fistula development, especially with type B or C fistula [46]. The associated treatment costs in these patients are 4–5 times higher than in patients without fistulas, highlights the socio-economic dimension of the health care system [8].
Management of Pancreatic Fistula
Regardless of the cause or the location of the pancreatic fistula, the steps required for treatment of a clinically relevant pancreatic fistula are similar. First, stabilization of patients and medical optimization are the crucial steps. Drainage of collections and insuring operatively placed drains are adequately controlling the fistula output to control sepsis that is mandatory. In cases with sepsis or high output fistulas, the patient is made “nil per os” (NPO) and parenteral nutrition is considered necessary. Only then should the nature of pancreatic duct injury be investigated and definitive management of the fistula be addressed.
Initial Management
The type of initial management needed for patients depends on the type of classified fistula and severity of symptoms. A clinically uncomplicated postoperative Grade A fistula can usually be managed by drainage alone, via intraoperatively placed drains which are still in situ and kept as long as necessary. Usually within 2–4 weeks, one sees spontaneous closure of the fistula. Fistula output volume and inflammatory parameters including white blood cell (WBC) should be monitored to avoid unrecognized fluid collections causing infectious complications despite continuing drainage.
< div class='tao-gold-member'>
Only gold members can continue reading. Log In or Register to continue
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree








