BMI <18 kg/m2
Weight loss >10 %
Low SGA score
Nonstressed state serum albumin <3.0 g/dL
Initiation of Nutritional Support
Key Concept: Although relatively healthy patients may safely wait several days prior to initiation of therapy, at–risk patients or those with moderate insults achieve optimal outcomes with early implementation of nutritional support.
For the well or mildly malnourished patient with an ongoing moderate systemic inflammatory response, initiating invasive feeding 5–7 days, or even more conservatively 7–10 days, after a period of fasting (if oral intake has not resumed) is a reasonable and well-accepted guideline. These principles reflect evidence that up to 10 days of routine fluid and electrolyte therapy can be tolerated without clinical harm in this circumstance [16]. Those with a poor premorbid nutritional status (i.e., more than 10 % weight loss or BMI less than 18 kg/m2) and superimposed illness are not as able to mount an adequate inflammatory response, which primarily impairs wound healing and immune function. This nutritionally related impairment of protein synthetic capacity increases susceptibility to infectious complications and organ failure. It is therefore important not to delay nutritional therapy and to intervene within the first several days. Although it has been shown to be more effective to feed moderately malnourished individuals for 7 days prior to an elective major operative procedure [17], present day reality is that preoperative or early post-injury feeding is generally not the rule. This practice needs to be reconsidered. For those with the most severe injuries which increase resting energy expenditure by 50 % or more, such as multiple trauma, major burns, closed head injury, and severe sepsis, all of which commonly result in lean tissue losses of 600–900 g/day without feeding, early feeding is essential. In this group of patients, even in the absence of pre-illness weight loss (rarely encountered by the colorectal surgeon), adjuvant nutritional therapy should be started soon after the acute resuscitation and metabolic issues are resolved. In this setting, early feeding may diminish the intensity of the systemic inflammatory response [18, 19], which could be an important factor in improved clinical outcome. Those patients who fall in between these extremes are the most challenging with regard to delivery choice and timing, and hopefully this chapter will better arm the reader regarding the role and benefits of nutritional support.
Nutritional Options: Enteral and Parenteral
Key Concept: Nutritional supplementation has a storied history that encompasses several unique attempts to help patients improve their status.
The goals of nutritional provision should be to provide adequate protein (at least 1 g/kg/day and optimally 1.5 g/kg/day) and energy (at least 1,000 kcal/day and optimally 25 kcal/kg/day) along with all essential nutrients, so as to allow optimal protein synthesis for the support of the immune system, wound healing, and vital organ function. Fortunately, there are two available options, enteral and parenteral nutrition, to reach these goals in essentially all patients.
The administration of supplemental enteral nutrition dates back hundreds of years. The ancient Egyptians provided support with enemas of wine, milk, whey, wheat, and barley. In 1790, Hunter created an orogastric tube made of a whalebone probe covered with eel skin attached to a bladder pump. After an assassination attempt in 1881, President Garfield was kept alive for 79 days with every 4-h rectal infusions of peptonized beef, broth, and whiskey. Furthermore, in 1918, Anderson placed the first nasojejunal feeding tube.
The ability to provide intravenous nutrition support came from revolutionary work done at the University of Pennsylvania in the 1960s. In 1962, Rhoads began infusing high volume peripheral solutions followed by diuretics. In 1966, Dudrick, Vars, and Rhoads documented their ability to support normal growth and development of beagle puppies with TPN. Finally in 1968, Dudrick and Wilmore demonstrated that they were able to safely support growth and development of infant fed entirely by TPN. This milestone revolutionized the nutritional support of surgical patients and has had a transformational impact on perioperative morbidity and mortality that continues to this day.
Enteral Feeding
Key Concept: Although the dictum “Whenever possible, feed the gut” is likely accurate, there is very little data in human studies to back up its superiority over the parenteral route.
It is commonly stated that the administration of enteral nutrition is “more physiologic” and its absence results in gut mucosal atrophy and increased intestinal permeability, predisposing to bacterial translocation and increased rates of septic complications [20]. Experimental evidence confirms that mucosal atrophy occurs with short-term bowel rest in animals [21], but human studies to date do not support these findings. Remaining NPO over a short time course (up to 1 month) has no substantial effect on mucosal architecture [22], while chronic starvation and malnutrition in humans do result in changes in villous architecture [23].
Several investigators have looked at the potential clinical benefits attributable to enteral feeding. There exists a large cohort of animal studies showing that functional stimulation of the GI tract resulting in the release of hormonal, biliary, and pancreatic secretions prevents mucosal atrophy [24]. Enteral nutrients also improve intestinal blood flow, increase the systemic and local immune response, increase the secretion of IgA, and increase the production of trophic hormones. Although these findings become considerably more vague when humans are studied and clinical outcomes are evaluated, there is strong suggestive evidence for benefit of early feeding, particularly enteral, in the most critically ill [18, 19].
In 1997, Reynolds et al. sought to answer the question if early enteral feeding after major upper GI surgery modulates gut barrier function and decreases the risk of major infectious complications compared with bowel rest and parenteral nutrition [25]. According to previous studies, TPN had been associated with an exaggerated acute phase and metabolic response after injury or endotoxin challenge, a response that could be attenuated by enteral nutrition [26]. The investigators prospectively randomized 67 surgical patients to either 7 days of TPN or enteral feeding via operative jejunostomy tubes. They showed that there was no clinical benefit attributable to the enteral route of nutrient administration when compared to the parenteral route. Furthermore, intestinal permeability was equally increased postoperatively in both groups (measured by lactose-mannitol ratios and serum antiendotoxin core antibodies), but the degree was not influenced by the provision of enteral or parenteral nutrition. In addition, the magnitude of surgery-induced changes in the acute phase reactants, albumin and C-reactive protein, were not different between groups. Their results revealed that major surgery does profoundly influence gut barrier function, but there was no evidence that enteral nutrition modulated gut barrier function or that septic morbidity was altered.
Early Enteral Feeding Versus NPO
Key Concept: Early enteral feeding is likely not harmful, though has questionable evidence–based benefit compared to NPO for otherwise healthy patients undergoing surgery. Some data exists citing improved outcomes to early enteral feeding in the critically ill.
If there does not appear to be a significant difference in the stress response and clinical outcome between enterally and parenterally fed patients, then is there a difference between patients fed enterally and those fed not at all? This question was studied in an extremely convincing article conducted by Heslin et al. from the Memorial Sloan-Kettering Cancer Center [16]. The authors prospectively randomized 195 patients undergoing surgery for major upper gastrointestinal cancer. The enterally fed group was administered an immune-enhancing diet via a jejunostomy tube, begun on POD #1 and advanced to a goal of 25 kcal/kg. The control group was administered intravenous crystalloid solutions. It is important to note that the patients studied in this trial were not malnourished, with mean preoperative weight loss of only 6 % and initial serum albumin concentration of 4.0 g/dL. The results revealed no significant differences in postoperative complications, hospital stay, or mortality. The authors concluded that there is no benefit to early enteral feeding in postoperative general surgical patients who are not malnourished at baseline.
A meta-analysis [27] looked at the 11 prospective randomized controlled trials comparing the practice of early enteral feeding to maintaining patients NPO after elective gastrointestinal surgery. Their analysis of 837 patients concluded that (1) there is no clear advantage to keeping patients NPO after elective GI surgery and (2) early feeding may be of benefit in decreasing infections and shortening postoperative length of stay. A closer evaluation of their pooled data revealed that the mean length of hospital stay was only reduced by 0.84 days. Although there was an increase in “any type of infection,” when considered individually, there was no difference in the incidence of anastomotic dehiscence, wound infections, pneumonia, intra-abdominal abscess, or mortality. Today early enteral feeding is a key component to enhanced recovery after surgery (ERAS) programs routinely used following colon resection surgery.
In 2001, Marik and Zaloga performed another meta-analysis, this time looking at the 15 randomized controlled trials that compared early with delayed enteral nutrition in critically ill surgical patients [28]. A total of 753 patients were analyzed: early enteral nutrition was associated with a significantly lower incidence of infections (RR reduction 0.45) and reduced length of hospital stay (2.2 days). There were no differences in noninfectious complications or mortality. The authors concluded that their pooled data supports the practice of early initiation of enteral feeding but that their conclusion should be interpreted with caution because of the heterogeneity between studies.
In summary, it has been sufficiently demonstrated that the provision of nutrients is important to both support the immune system and heal surgical wounds. Investigators have further studied the route, timing, and metabolic response of nutritional support. Reasonable conclusions based on the studies quoted above would be that:
1.
There is no benefit from the immediate administration of enteral nutrition to patients who undergo routine general surgical procedures but are well nourished at baseline.
2.
When patients are either malnourished or in the post-injury stressed state, there is no obvious harm (and there may be a clinical benefit) to the initiation of immediate enteral feeding, particularly in the more critically ill patient.
3.
The specific clinical benefits of a more aggressive approach have yet to be confirmed and will likely require better powered future studies which focus on particular subsets of patients.
Enteral Shortcomings
Key Concept: The benefit of enteral feeding may be hindered by an inability to tolerate feeds with either a nasojejunal or nasogastric route.
While there appears to be support in the literature for early enteral nutrition, most especially with immune-enhancing formulas, the majority of studies are handicapped by the high prevalence of gastrointestinal intolerance leading to inadequate tube feed administration. In fact, several studies have shown that patients who rely on enteral feeding alone are underfed. In 1995, Heyland et al. enrolled 99 consecutive ICU patients and recorded the initiating time and tolerance of tube feeds [29]. They found that approximately one half of critically ill, hypermetabolic ICU patients were intolerant of enteral feeding due to gastrointestinal dysfunction. Subsequently, in 2001, De Jonghe followed 51 consecutive ICU patients for the first 14 days of nutritional delivery [30]. The investigators discovered inadequate routine delivery of enteral nutrition, with more than half of the study patients receiving <70 % of their nutritional goals. On further inspection, the etiology was multifactorial, equally distributed among the following: digestive intolerance (high gastric residual >200 cc, constipation, diarrhea, abdominal distention, vomiting, and regurgitation), airway management issues, and discontinuation for diagnostic procedures.
To address this well-defined problem of enteral feeding intolerance and resultant inadequate nutrition delivery, multiple studies have been performed to evaluate whether a potential change in clinical practice may improve outcome. When providing enteral nutrition, the decision to feed into the stomach or into the small bowel has been both a point of debate and of substantial clinical investigation. One approach has been to determine if there is a noticeable clinical difference when delivering enteral formulas into the stomach, into the duodenum, or into the jejunum. When discussing “transpyloric” or “postpyloric” feeding, most investigators distinguish between delivering nutrients (1) into some portion of the duodenum or (2) beyond the ligament of Treitz, into the proximal jejunum. The hypothesis has been that in bypassing the region of potential gastroduodenal dysmotility, nutrients directly infused into the jejunum would improve tolerance while decreasing the potential for feeding complications such as aspiration (Fig. 3.1).

Fig. 3.1
Postpyloric positioning of nasoenteric feeding tube
Aspiration
When investigators have prospectively compared nasogastric to nasoduodenal feeding in principally medical ICU patients, there has been no evidence for decreased rates of aspiration [31] or of aspiration pneumonia [32–34]. Similar findings have been noted when comparing nasogastric to nasojejunal feeding [35–37]. This is likely due to the lower esophageal sphincter being stented open by any tube, regardless of where its tip is positioned.
Esparza et al conducted a trial in 2001 which randomized 54 ICU patients to either gastric or transpyloric feeding [31]. All feeds were tagged with technetium 99m-radiolabeled sulfur colloid, and the pulmonary secretions or lungs were scanned on a daily basis to determine whether aspiration had occurred. There was a nonsignificant difference in aspiration between the gastric and transpylorically fed patients (7 % vs. 13 %). These findings suggest that regurgitation of postpylorically delivered feeds exists secondary to retrograde peristalsis but does not result in an increase in actual aspiration or in clinically definable pneumonia.
Feeding Tolerance
Key Concept: Nasogastric and nasojejunal routes have similar rates of feeding tolerance.
The medications that have been employed as prokinetic agents include antidopaminergic agents (i.e., metoclopramide), serotonergic agents (i.e., cisapride), and motilin receptor agonists (i.e., erythromycin). While there is some support for a role in the setting of gastroparesis, these agents have been proven useless in impacting the degree or duration small bowel or colonic ileus [38]. In addition to lack of efficacy, administration of many of these agents results in unacceptable side effects. Cisapride is no longer available due to cardiac arrhythmias and risk of sudden death. The practicing surgeon commonly adds such agents with a hope of stimulating GI functional recovery, often understanding the lack of benefit while under recognizing the potential risks. These agents should not be used at present, as we await new formulations with more reliable and predictable results. Alvimopan is a peripheral μ antagonist that may prophylactically prevent postoperative ileus but must be initiated prior to narcotic administration.
Regarding the issue of feeding tolerance, several studies do reveal significantly greater nutrient delivery when feeding beyond the pylorus [32, 34] while others do not [33, 35–37]. This difference appears to be primarily related to the practice of holding feeds for high gastric residuals, the most frequent gastrointestinal complication associated with enteral feeding leading to decreased nutritional intake [39]. When a more aggressive feeding protocol is followed, nasogastrically fed patients, despite having higher gastric residual volumes, receive equivalent amounts of enteral nutrition to those fed nasojejunally [35].
One of the earliest studies that evaluated the potential difference between intragastric and jejunal feedings was performed in 1992 by Montecalvo et al. [40]. They prospectively randomized 38 ICU patients to receive feeds through either gastric or endoscopically placed jejunal tubes. Those patients fed by the jejunal route received a significantly higher proportion of their goal caloric intake (46.9 % vs. 61 %, p < .05) but had equal rates of pneumonia (0 % vs. 10 %, p = NS).
In 2002, Davies et al. performed a prospective, randomized trial to evaluate the potential benefits of nasojejunal (NJ) feeding compared to nasogastric (NG) feeding [35]. By feeding directly into the jejunum, the authors hypothesized that the patients would be more tolerant of enteral nutrition. The study distinguished between criteria for “ceasing” tube feeds and criteria for declaring a patient “intolerant” of feeds. Instead of using the traditional cutoff range for high gastric residual volumes (150–200 cc), the authors continued feeds until a residual measured >250 cc beyond the previous residual measurement. They also commenced feeds at a rate of 20 cc/h and aggressively advanced by 20 cc every 4 h. Patients were only declared “intolerant” when, over a 48-h period, (1) feeds were stopped four times due to one of their predefined complications or (2) a total gastric residual volumes of 2,000 cc was reached. By these criteria, 4/31 patients in the NJ group were intolerant, although all 31 were eventually tolerant of enteral feeds after a holding period. A total of 11/35 patients in the NG group were intolerant. Of the 11 who were intolerant, 10 were eventually tolerant, either by NG or NJ feeding. Only 1 patient went on to TPN.
Although the authors did demonstrate a significant difference in gastric residual volumes (incidence 32 % vs. 74 %), there was no difference in feeding tolerance. Once feeding was initiated, there was no difference between the groups with regard to the volume delivered at 24 and 48 h or the time to reach target rate. Also, there were no differences in clinical complications such as bleeding, pneumonia, sepsis, SIRS, or mortality. It should be noted that there was a 1-day delay in initiating enteral feeding in the NJ group. This was directly attributable to a scheduling delay required to arrange endoscopy by the gastroenterologist for tube placement. The endoscopic placement of jejunal tubes does appear to be technically feasible (98 % success rate) and safe but is the effort and expense worth the trouble? As the enteral feeding tubes were inadvertently removed in approximately one-third of the patients, tube dislodgment remains a major problem and is likely a major factor in reaching goal nutrition. NG tubes can be replaced at the bedside, but NJ tubes replacement typically requires repeat endoscopy or radiologic assistance.
Although NJ feeding results in reduced gastric residual volumes, this feeding method does not consistently improve feeding tolerance. It can be helpful in certain subsets of patients, suggesting that NJ feeding can be an alternative to TPN in the patient proven or likely to be intolerant to NG feeding. This is an important finding. Its applicability to populations with higher likelihood of gastrointestinal intolerance such as postoperative patients or those with severe pancreatitis or closed head injury will require further study before implementation as a standard for all patients.
Enteral Complications
Key Concept: Though perhaps not as well publicized, enteral feeding has its own unique set of potential complications surgeons should be aware of.
One of the arguments used in support of enteral over parenteral feeding revolves around the morbidity associated with central venous line placement and maintenance. In fact, enteral feeding practices can often lead to unique adverse effects of their own such as high gastric residuals leading to reflux, emesis and aspiration, abdominal distention, diarrhea, constipation, and rarely mesenteric ischemia. Mechanical complications include misplacement (endobronchial, intrapulmonary, and transesophageal), dislodgement, or malfunction from luminal blockage. Finally, enteral feeding (particularly of ICU patients) is regularly discontinued for both diagnostic and interventional procedures, often resulting in patient underfeeding (Fig. 3.2).
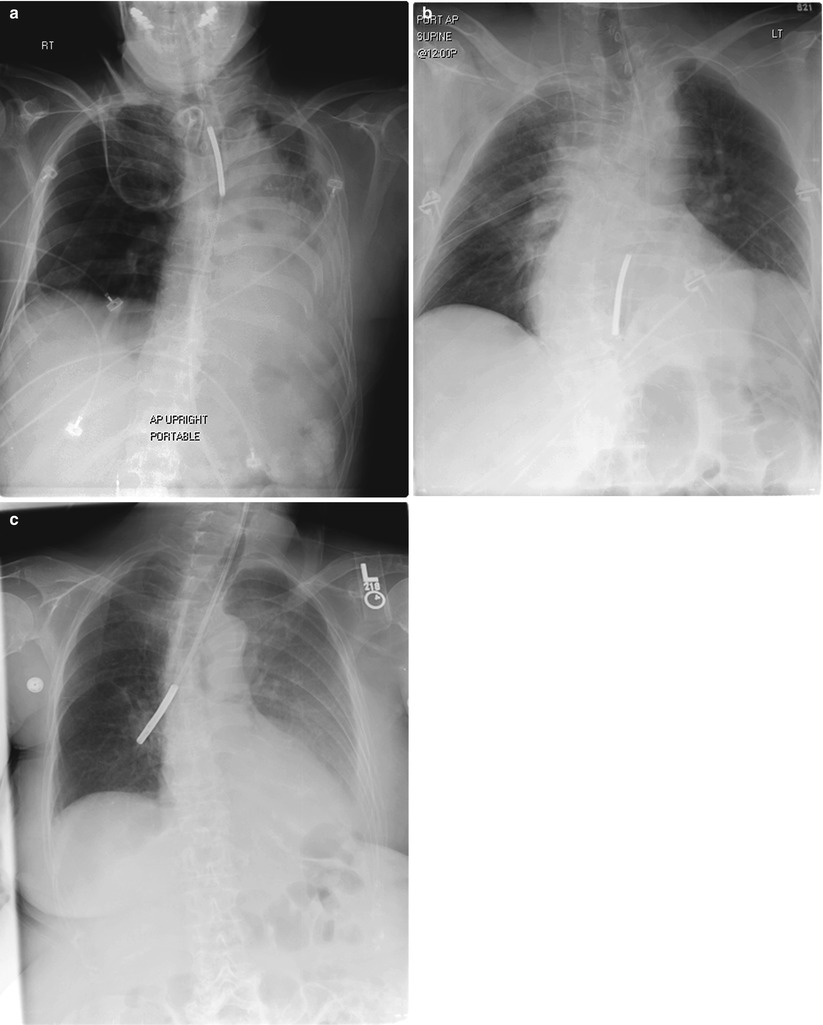

Fig. 3.2
Malpositioned nasoenteric feeding tubes, highlighting the importance of verifying tip location prior to initiation of feeds. (a) Coiled tip in proximal esophagus (b). Tip in mid-esophagus (c). Tip in right mainstem bronchus
The above complications become particularly important when one attempts to bypass the physiologic brake of gastroduodenal dysmotility by placing a postpyloric feeding tube. There is a very small but substantial risk of mesenteric ischemia associated with jejunal feeding (estimated at 1 in 1,359), which is most likely to occur in patients showing signs of abdominal pain, distention, increased NG drainage, or intestinal ileus [41]. Given this uncommon but serious potential complication with NJ feeding, protocols to avoid high-risk patients such as hypotensive patients receiving pressor agents or those with significant abdominal pain seem appropriate, since its low incidence renders this complication outside the ability to study clinically. Fortunately, feeding-related small bowel necrosis is not encountered by most surgeons or intensivists, but this complication carries a significant mortality (86 %).
Total Parenteral Nutrition
Key Concept: Underlying problems with the available literature regarding perioperative TPN use make widespread reliable conclusions difficult. Surgeons should be aware of proper formulation and practice guidelines, as well as the potential complications that can arise with the use of TPN.
Unfortunately, the literature that evaluates the role for parenteral support is typically old, employing outdated practices and generally of low quality due to heterogeneous patient populations, variable study designs, and excessive feeding protocols. Early studies and subsequent meta-analyses have revealed conflicting results.
Von Meyenfeldt et al. published one of the earliest randomized controlled trials evaluating the impact of preoperative TPN on postoperative morbidity [42]. The authors randomized 50 patients to 10 days of preoperative TPN and 50 patients to 10 days of preoperative enteral nutrition. These patients were compared to a group of 50 malnourished control patients. The investigators observed a significant decrease in postoperative complications in patients who administered preoperative nutrition and were high risk (>10 % weight loss and >500 cc blood loss) (p < 0.05).
In 1997, an expert committee reviewed all published studies evaluating the use of TPN in the perioperative setting [43]. When delivered preoperatively, TPN appeared to decrease the risk of postoperative complications by 10 %, yet no difference in mortality was noted. When delivered postoperatively, TPN was found to increase postoperative complications by 10 %, again with no mortality difference. Even at the time the authors recognized that the type and quantity of nutrients delivered were suboptimal and calories were given in excess of metabolic needs, potentially impacting outcomes.
In 2000, Bozzetti et al. looked at the role of perioperative TPN in malnourished gastrointestinal cancer patients. 90 patients with gastric or colorectal tumors and >10 % weight loss were randomized to 10 days of preoperative and 9 days of postoperative nutrition vs. control group [44]. The TPN group suffered fewer postoperative complications 37 % vs. 57 % (p = 0.03) and fewer deaths (0 vs. 5, p = 0.05.). Even though the patients were overfed at >35 kcal/kg, the overall and infectious complications were fewer, likely as a result of appropriate selection of highest risk, malnourished patients.
Complications
Catheter
The placement of a central venous catheter is necessary for the initiation of total parenteral nutrition. The hyperosmolar solution must be delivered into the large diameter, high-flow vena cava to prevent phlebitis seen when delivered into the peripheral veins. A PICC line is currently the most common form of access, supplanting the triple lumen placed at the internal jugular or subclavian location. While considered safer with less risk of pneumothorax or injury to the chest vessels, PICC placement is not benign and can still be complicated by misplacement, dislodgment, cardiac arrhythmias, thrombosis, and infection. Long-term tunneled lines may be more resistant to infection, though are more invasive with their own set of complications. All catheters are at risk for infection, and this is a direct consequence of local care and access technique. Thrombosis can complicate lines in the upper extremities due to intraluminal thrombus or fibrin clot at the catheter tip. If a catheter-related thrombosis is diagnosed, the catheter should be removed and the patient systemically anticoagulated. Less common complications include pneumothorax, vascular injury, air embolism, thoracic duct injury, brachial plexus injury, and catheter erosion.
Metabolic
TPN should be considered a compounded medication and therefore requires an understanding of its components and risks of administration. While some institutions have “standardized” formulas or even nutrition support teams to guide the prescription process, many surgeons are tasked with writing a customized solution on a daily basis. Inappropriate formulation can result in electrolyte abnormalities (commonly including potassium and magnesium) as well as acid–base disturbances. Excess calcium or phosphorus can lead to precipitation. Excess water can cause hyponatremia. One of the most important issues related to TPN administration surrounds glycemic control. Excessive dextrose administration, particularly to a diabetic patient or those on steroids, can iatrogenically create a state of hyperglycemia with significant impact of patient morbidity.
How to Write TPN
Key Concept: Matching the TPN formulation with the individual patient’s needs is an obvious, yet understated (and underperformed), critical aspect to better outcomes.
This review of potential complications of TPN prompts a review of a safe way to initiate and advance TPN. While each institution varies regarding TPN formula options and compounding process, this review will highlight some of the salient points that help the practitioner prescribe TPN in a systematic and safe fashion (Table 3.2).
Table 3.2
Systematic approach to the safe and appropriate prescription of TPN (may vary based on institution’s products and protocols)
Initial calculations | |
1. Determine the “feeding weight”: | |
Calculate ideal body weight (IBW) | |
Men: 106 lb for 1st 5 ft and 6 lb for each inch thereafter | |
Women: 100 lb for the 1st 5 ft and 5 lb for each inch thereafter | |
Compare to actual body weight (ABW) or usual body weight (UBW) | |
If a big discrepancy, calculate adjusted feeding weight | |
If patient is underweight, generally use IBW | |
If patient is obese (>120 % IBW), then add 25 % of the difference between the ABW and IBW to the IBW | |
Amputations | |
IBW less 6 % for BKA | |
IBW less 9 % for AKA | |
Using the example of a 70 kg person (“feeding weight”): | |
2. Calculate GOAL nutritional support. Usually | |
(a) Protein: 1.5 g/kg/day (1.5 × 70 = 105 g) | |
(b) Kilocalories: 25 kcal/kg/day (25 × 70 = 1,750 kcal) | |
3. Determine the components of the GOAL TPN admixture (no lipids/2:1) | |
(a) Start with total kilocalories | |
1,750 kcal | |
(b) Calculate how much of total kcal will come from goal protein | |
105 g × 4 kcal/g = 420 kcal | |
(c) Subtract this amount of calories from the goal/total | |
1,750–420 = 1,330 kcal. | |
(d) Make up the difference with dextrose | |
1,330 kcal ÷ 3.4 kcal/g = 390 g dextrose | |
4. Determine the components of the GOAL TPN admixture (with lipids/3:1) | |
(a) Start with total kilocalories | |
1,750 kcal | |
(b) Calculate 20 % (or 30 %) of the total calories, and provide this as lipids | |
1,750 × 0.2 = 350 kcal | |
350 kcal ÷ 9 kcal/g = 38 g (may round off to 35 g lipids (so lipids actually provide 315 kcal))
Stay updated, free articles. Join our Telegram channel
Full access? Get Clinical Tree
 Get Clinical Tree app for offline access
Get Clinical Tree app for offline access

|





