This concept of a Doppler shift is used to measure blood flow velocity whereby the shift in sound-wave frequency is detected by the ultrasound transducer after encountering active blood flow.
However, several factors influence the resultant frequency shift and hence the measured velocity. These include the incident frequency of the ultrasound beam used, speed of sound in soft tissues, the velocity of the moving reflectors (i.e., blood in a vessel), and the angle between the incident beam and vector of blood flow (θ) called the angle of insonation.
The angle of insonation is inversely related to Doppler shift. Hence, as the angle of insonation increases, approaching 90°, the Doppler shift decreases, and therefore, the calculated blood flow velocity decreases to 0. The Doppler angle is therefore a significant technical consideration in performing duplex Doppler examinations, and an ideal angle of insonance between 0 and 60° is required (Fig. 1).
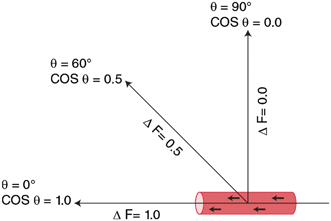
Fig. 1
Doppler angle: The change in Doppler frequency (ΔF) is directly related to the cosine of the angle of insonance (θ). The angle of insonance (the angle between the incident beam and the vector of blood flow) must be less than 60° for accurate measurements of blood flow velocity
Clinical Pearl: Even if the angle of insonance is not corrected, the RI will be accurate. However, PSV and EDV will be inaccurate.
Patient Preparation
The patient should lie comfortably on the examination table in a supine position with legs together providing support for the external genitalia. An alternative position is dorsal lithotomy with the penis lying on the anterior abdominal wall. Regardless of the patient position preferred, the area of interest should remain undraped for the duration of the examination. Care should be taken to cover the remainder of the patient as completely as possible including the abdomen, torso, and lower extremities. Ample amounts of ultrasonographic acoustic gel should be used between the transducer probe and the surface of the penis to allow uninterrupted transmission of sound waves, thus producing a high quality image without acoustic interruption.
Penile Ultrasound Protocol
As with other ultrasound exams, penile ultrasound uses specific scanning techniques and images targeting the clinical indication prompting the study. Irrespective of the indication for penile ultrasound, routine scanning during penile ultrasound should include both transverse and longitudinal views of the penis by placing the transducer probe on the dorsal or ventral aspect of the penis. The technique presented here, uses a dorsal approach, which is easier for the flaccid phallus. However, the ventral approach, often with placement of legs in the lithotomy position, is often better with a fully erect phallus as well as being able to visualize the proximal corpora cavernosa. The goal is to visualize the cross-sectional view of the two corpora cavernosa dorsally and the corpus spongiosum ventrally along the length of the penis from the base of the penile shaft to the glans penis (Fig. 2).
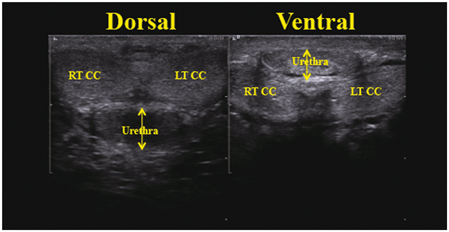
Fig. 2
A transverse view of the phallus with the transducer placed either on the dorsal or ventral surface. Note the compression of the urethra and corporal spongiosum compression in the ventral projection with minimal pressure applied to the phallus. (Campbell’s Urology, Fig. 3-40)
The corpora cavernosa appear dorsally, as two homogeneously hypoechoic circular structures, each surrounded by a thin (usually less than 2 mm) hyperechoic layer representing the tunica albuginea that envelops the corpora. The corpus spongiosum is a ventrally located circular structure with homogeneous echotexture, usually more echogenic than the corpora cavernosa [1]. It is best visualized by placing the ultrasound transducer probe on the ventral aspect of the penis, however, the urethra is easily compressible so minimal pressure should be maintained while scanning. For routine anatomic scanning of the flaccid penis with ultrasound, all three corpora can be sufficiently viewed from a single dorsal approach to the penile shaft. A survey scan is first performed prior to obtaining static images at the proximal (base), mid-portion, and distal (tip) of the corpora cavernosal bodies for documentation (Figs. 3–5). The value of the survey scan cannot be over stated. It often provides the prospective that is necessary to assure absence of coexisting pathology. A careful survey scan of the phallus will identify abnormalities of the cavernosal vessels, calcified plaques and abnormalities of the spongiosa tissue.
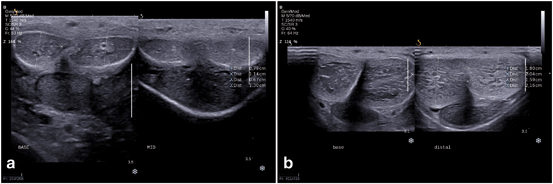
Fig. 3
a Flaccid phallus survey scan with transverse views through the base (left panel) and midshaft (right panel) of the penis. In this image, the transducer is on the dorsal penile surface and demonstrates the right and left corpora cavernosa nearer the ultrasound probe and corpus spongiosum in the midline ventrally, furthest from the ultrasound probe. b Pharmacologically stimulated phallus survey scan with transverse views through the base (left panel) and distal shaft (right panel) regions of the penis. Similarly, in this image, the transducer is on the dorsal penile surface and demonstrates the right and left corpora cavernosa dorsally (closest to the ultrasound probe) and urethra ventrally (away from the ultrasound probe)
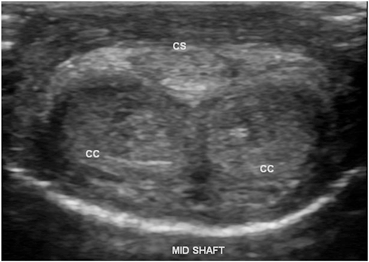
Fig. 4
Normal mid-shaft view with the ultrasound transducer on the ventral surface of the phallus depicting the right and left corpora cavernosa (CC) and corpus spongiosum (CS)
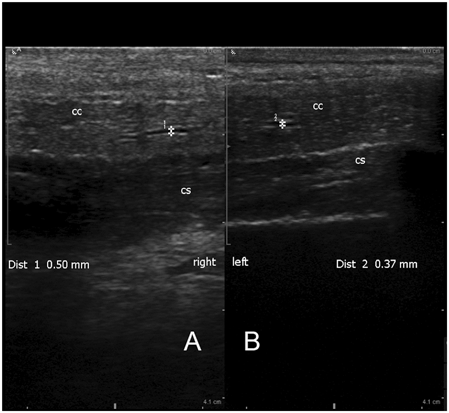
Fig. 5
a Color Doppler and spectral ultrasound findings in a high-flow priapism demonstrating high peak systolic and high-end diastolic velocity in the cavernosal artery feeding the arteriovenous fistula (AVF). b Color Doppler and spectral ultrasound findings in a low-flow priapism demonstrating a negative end diastolic diastolic velocity (i.e., reversal of flow) and a resistive index of 1.19. Subjective tumescence was 100 % with rigidity of 95 %
Still images recommended as representative views of this initial survey scan of the flaccid phallus include one transverse view at the base of the penile shaft, one at the mid-shaft, and a third at the distal shaft just proximal to the corona of the glans penis (Fig. 3a). Each image should show transverse sections of all three corporal bodies. As noted in the labeled images, orientation by convention is for the right corporal body to be on the left side of the display (as viewed by the sonographer) while the left corporal body is located on the right side of the display on images obtained with the ultrasound probe on the dorsal aspect of the phallus. Although performed as an initial survey scan in the flaccid phallus, this can also be after pharmacostimulation for comparison (Fig. 3b). Figure 4 demonstrates a normal mid-shaft view with the transducer on the ventral aspect of the phallus. A longitudinal projection splitting the screen view helps to compare the right and left corporal bodies. Figure 5 demonstrates a dorsal approach with measurements of the cavernosal artery diameter. By convention, the orientation is constant, with the projection of the right corporal body on the left side of the display while the left corporal body is located on the right side of the display.
Focused Penile Ultrasound by Indication
There are several accepted indications for penile ultrasound, each with specialized focus beyond the routine survey scan as previously described. General guidelines for the use of penile ultrasound are delineated by the “Consensus Statement of Urologic Ultrasound Utilization” put forth by the American Urologic Association [2] and the American Institute for Ultrasound in Medicine (AIUM). These indications can be further classified as either vascular, structural, or urethral pathology in nature (Table 1).
Table 1
Indications for penile and urethral ultrasound
Vascular Pathology |
Erectile Dysfunction (ED) |
Cavernosal Artery Diameter |
Flow velocity |
Peak systolic velocity (PSV) |
End diastolic velocity (EDV) |
Resistive Index (RI) |
Priapism |
High-flow (arterial) |
Low-flow (ischemic) |
Penile Trauma/Fracture |
Dorsal Vein Thrombosis |
Structural Pathology |
Penile Fibrosis/Peyronie’s Disease |
Plaque assessment (number, location, echogenicity and size) |
Perfusion abnormalities |
Perfusion surrounding plaques |
Penile Mass |
Primary penile tumors |
Metastatic lesions to the penis |
Penile Foreign Body (size, location, echogenicity) |
Penile Urethral Disease |
Urethral stricture (location, size) |
Perfusion surrounding plaques |
Calculus/Foreign Body |
Urethral diverticulum/cyst/abscess |
Erectile Dysfunction
PDU has been a vital part of the assessment of patients with ED . Some practitioners immediately turn to intracavernosal injection therapy with vasoactive agents in patients who have failed a course of oral phosphodiesterase-5 inhibitors. However, PDU may be used as a diagnostic tool in conjunction with commencement of injection therapy. PDU allows for a baseline evaluation of the functional anatomy as well as providing a real-time assessment of the dynamic changes experienced in response to the dosing of vasoactive medications. In cases where intracavernosal injection of vasoactive substances does not prompt a penile erection, documentation provided by PDU will be a foundation for other management options including use of vacuum constriction devices or insertion of a penile prosthesis .
Possibly one of the most compelling reasons for the performance PDU in men presenting with ED is the finding that impaired penile vascular dynamics, as documented on PDU, may be associated with a generalized vessel disease that often predates cardiovascular disease by 5–10 years [3–5]. Significantly, early treatment of metabolic factors (e.g., hypertension, dyslipidemia, hyperglycemia) can delay and possibly prevent the development of cardiovascular disease [6, 7]. Therefore, the physician evaluating ED has a unique opportunity to diagnosis vascular impairment at a time when lifestyle changes and possible medical intervention have the potential to change morbidity and mortality of cardiovascular disease. As suggested by Miner, there might be a “window of curability” in which the significant risk of future cardiovascular events might be averted through early diagnosis and treatment [8–10].
In cases of diagnostic study for ED, emphasis is directed toward the cavernosal arteries. However, the initial survey scan is essential to evaluate for plaques, intravernosal lesions and urethral pathology as well as evaluation of the dorsal penile vessels. The cavernosal arteries are visualized within the corpora cavernosa, and the depth of these arteries can be easily defined within the corpora during transverse scanning to ensure a comprehensively represented assessment of diameter at different points along its course. Color Doppler examination of the penis should be performed in both transverse and longitudinal planes of view. Using the transverse views as a guide to cavernosal artery depth, turning the transducer probe 90° then provides longitudinal views of each corpus cavernosum separately, allowing for identification of the cavernosal arteries in longitudinal section (Fig. 5). The diameter of the cavernosal artery should be measured on each side. Color flow Doppler makes recognition of the location and direction of blood flow easy. Measurements of vessel diameter to assess the peak systolic flow velocity (PSV) as well as end-diastolic flow velocity (EDV), allow for the assessment of a vascular resistive index (RI) (Fig. 6) . The diameter of the cavernosal artery ranges from 0.2 to 1.0 mm in a flaccid penis [11, 12]. PSV varies at different points along the length of the cavernosal artery, typically with higher velocities occur more proximally [13]. Hence, the assessment of the PSV and EDV should be recorded at the junction of the proximal one-third and the distal two-thirds of the penile shaft. In the flaccid state, cavernosal artery PSV normally measures 5–15 cm/s, at baseline. This should be assessed and compared to the pharmacostimulated state [14, 15].
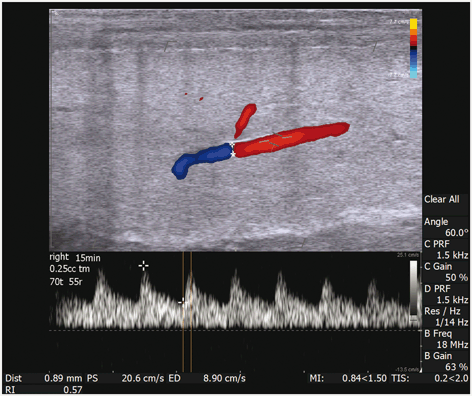
Fig. 6
The right cavernosal artery is imaged 15 min after intracavernosal injection of 0.25 mL of trimix solution. The measured vessel diameter is 0.89 mm. The direction of flow and a dorsal branch of the cavernosal artery is easily appreciated with color Doppler. Also, documented on this image is the measurement of arterial diameter (0.89 mm), PSV (20.6 cm/s), EDV (8.9 cm/s), and calculated RI (0.57) are shown. Please note that the angle of incidence is electronically made to be 60° by both electronic steering of the transducer and aligning the cursor to be parallel to the flow of blood through the artery. In addition the width of the caliper is adjusted to be approximately 3/4 the width of the artery for best sampling
The intracavernosal injection should then be given. An overview of the injection procedure that we teach our patients is shown in the appendix. At regimented serial time points following the injection of vasoactive medication, cavernosal artery dimensions and flow velocities should be recorded to assess the response to pharmacologic stimulation. After prepping the lateral aspect of the penile shaft with an alcohol or providone-iodine prep pad, a finely measured volume of a vasoactive agent should be injected into one corpus cavernosum (in the distal two-thirds of the penile shaft) using a 29, or 30 gauge 1/2 inch needle. Pressure should be held on the injection site for at least 2 min to prevent hematoma formation. The amount to inject is patient specific. For example, a patient presenting with no erections after a radical prostatectomy that had normal erections prior to his procedure would be given a very low dose (i.e., 0.05 mL) of our standard TriMix (Papaverine 30 mg/mL; Phentolamine 2 mg/mL; PGE-1 10 mcg/mL). A patient however, with significant cardiovascular disease with no erections would be given a much higher dose to begin with (i.e., 0.2 mL or greater) .
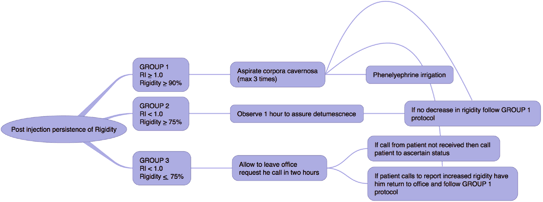
Vasoactive agents used for pharmacologic stimulation of erection include prostaglandin E1, papaverine, or trimix (combination of prostaglandin E1, papaverine, and phentolamine) [16]. As with every medication administration, the expiration date of the medication should be reviewed, patient allergies should be evaluated, and the dosage administered should be documented. We obtain an informed consent after the patient is counseled about the known risk for developing a low-flow priapism and appropriate follow-up if this were to arise [17]. This protocol requires the patient to stay in the office until penile detumescence occurs. A treatment protocol for low-flow priapism is given in Table 2. Of note, for patients in which we have given a vasoactive agent and have had to treat for low-flow priapism, aspiration, irrigation, and injection of intracorporal phenylephrine are usually successful to reverse the priapism state. In our experience, corporal aspiration alone has been uniformly successful in the setting of pharmacologically induced priapism in the absence of confounding factors (e.g., concomitant use of phosphdiesterase inhibitors, sickle cell disease, etc.) following diagnostic duplex penile ultrasonography .
Table 2
Treatment protocol for low-flow priapism caused by pharmocologic induction by vasoactive agents
1. Observation: If no detumescence in 1 h, then |
2. Aspiration: With a 19 or 21 gauge butterfly needle aspirate 30–60 cc corporal blood. Repeat in 1/2 h if 100 % rigidity returns. May be repeated up to three times. If 100 % rigidity persists than consider pharmacologic detumescence |
3. Pharmacologic detumescence: a. Phenylepherine 100–500 mcg injected in a volume of 0.3–1 cc every 3–5 min for a maximum of 1 h b. Monitor for acute hypertension, headache, reflex bradycardia, tachycardia, palpitations, and cardiac arrhythmia c. Serial noninvasive blood pressure and continuous electrocardiogram monitoring are recommended |
Our algorithm for persistence of rigidity after an in office diagnostic injection:
Arteriogenic ED is a form of peripheral vascular disease, commonly associated with diabetes mellitus and/or coronary artery disease. PSV is the most accurate measure of arterial disease as the cause of ED. The average PSV after intracavernosal injection of vasoactive agents in healthy volunteers without ED ranges from 35–47 cm/s, with a PSV of 35 cm/s or greater signifying arterial sufficiency following pharmacostimulation [18–23]. Primary criteria for arteriogenic ED include a PSV less than 25 cm/s, Cavernosal artery dilation less than 75 %, Acceleration time > 110 ms. In cases of equivocal PSV measurements, particularly when PSV is between 25 and 35 cm/s include, we look for asymmetry of greater than 10 cm/s in PSV comparing the two cavernosal arteries, focal stenosis of the cavernosal artery, cavernoal artery and cavernsal-spongiosal flow reversal [24].
Veno-occlusive insufficiency, also referred to as venous leak, can only be diagnosed in cases of ED where the patient was confirmed to have appropriate arterial function as measured by PSV. PDU parameters to assess the presence of veno-occlusive insufficiency as the cause of ED are EDV and RI. Antegrade EDV greater than 5 cm/s in the cavernosal artery demonstrated throughout the study, especially at the most turgid level of erection achieved, is suggestive of a venous leak [25, 26]. This is only true if PSV is normal. Arteriogenic dysfunction by definition fails to produce a fully tumescent and rigid phallus. In the setting of venous leak, EDV is always greater than 0. The definitive test for venous leak is the DICC (dynamic infusion cavernosography and cavernosometry). However, when both arteriogenic and venogenic dysfunction exists, interpretation of DICC is difficult. On PDU, an RI of less than 0.75, measured 20 min following maximal pharmacostimulation has been found to be associated with a venous leak in 95 % of the patients [27]. In the absence of a venous leak, a fully erect penis should have an EDV nearing zero and hence, the RI should approach or exceed (when reverse flow occurs) 1.0 (Fig. 7). In cases of diagnostic PDU with intracavernosal pharmacostimulation where a RI of 1.0 or greater is achieved, we recommend immediate treatment or prolonged observation to achieve detumescence because of the high specificity of absent diastolic flow for priapism [28].
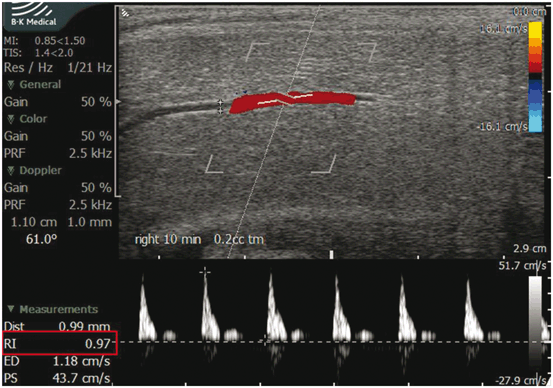
Fig. 7
In a fully erect phallus, the RI should approach or exceed 1.0. If this condition persists it is termed as low-flow priapism. Color Doppler ultrasound findings in low-flow priapism demonstrate poor flow or absent flow in the cavernosal artery of the penis with moderate flow in the dorsal artery and vein
In cases where arterial function and venous leak may be coexistent processes, indeterminate results may be yielded on PDU and a mixed vascular cause of ED may be assumed. However, venous competence cannot be accurately assessed in a patient with arterial insufficiency (Fig. 8).
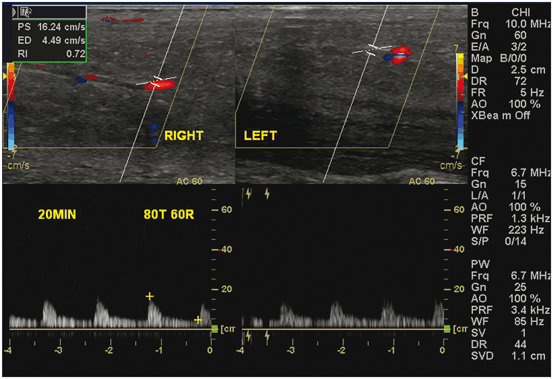
Fig. 8
With maximal stimulation, a PSV less than 25 cm/s suggests significant arteriogenic dysfunction. In this patient, injection with a maximal dose of a mixture of papaverine, phentolamine and proitaglandin E1, the peak systolic (PS) velocity was 16.24 cm/s with an elevated end diastolic velocity of 4.49 with a calculated resistive index of 0.72. When a maximal PS velocity is less than 25 cm/s, referral for evaluation of cardiovascular disease is recommended
As previously discussed, arteriogenic ED has been found to correlate directly with other systemic cardiovascular diseases, both coronary artery disease (CAD) and peripheral vascular disease (PVD), in a number of population studies [29, 30]. Researchers have postulated the common risk factor of atherosclerotic vascular disease and impaired endothelium-dependent vasodilation by way of the nitric oxide pathway as the underlying pathophysiologic explanation for the remarkable overlap between these disease processes [31–33]. Also, hypogonadism has been noted as a common etiology for organic ED and disorders leading to metabolic syndrome [34, 35]. Vessel compliance is compromised in arteriogenic ED as it is in CAD. Patients with severe vascular etiology ED, have an increased cavernosal artery diameter of less than 75 % (with overall luminal diameter rarely above 0.7 mm) following injection of vasoactive agents into the corpora cavernosa [22, 36].
Studies have demonstrated that vasculogenic ED may actually provide a lead-time on otherwise silent and undiagnosed cardiovascular disease [29, 37, 38]. ED has also been found to predict metabolic syndrome in men with normal body weight, as defined by body mass index (BMI) less than 25 kg/m2, suggesting that the early diagnosis and intervention of vasculogenic ED might avert significant morbidity and provide a public health benefit by reducing the significant risk of cardiovascular and metabolic syndrome risk in men with ED [3, 5, 10, 39–42]. This is why we recommend referral for cardiovascular evaluation in men with a maximal PSV of less than 16 cm/s after injection of a maximal dose of a pharmacologic agent .
Priapism
Priapism can be differentiated as low-flow (ischemic) or high-flow (arterial) using PDU. Ultrasound plays an adjunct role to an illustrative history which may commonly indicate the likely underlying mechanism of priapism. Laboratory tests including a cavernosal blood gas, PDU provides documentable findings that may guide further treatment. High-flow priapism is commonly a result of pelvic or perineal trauma which results in arterial fistulization between the cavernosal artery and the lacunae of the corpus cavernosum. Unlike low-flow priapism, which is a medical emergency associated with severely compromised venous drainage from the corpora cavernosa, high-flow priapism does not result in venous stasis and rapid risk of tissue necrosis. Ultrasound used as an aide in the definitive diagnosis and localization of the cause of high-flow priapism can expedite treatment with selective angioembolization [43]. In cases of high-flow priapism PDU reveals normal or increased blood flow within the cavernosal arteries and irregular, turbulent flow pattern between the artery into the cavernosal body at the site of an arterial-lacunar fistula (Fig. 9a). In contrast, a low-flow priapism on PDU would present with absent or very high-resistance flow within the cavernosal artery (Fig. 9b).
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree








